ZEPBOUND Solution for injection Ref.[109319] Active ingredients: Tirzepatide
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
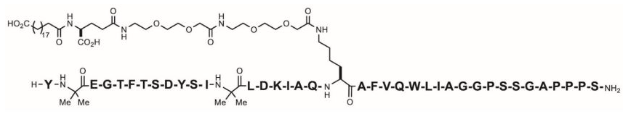
ZEPBOUND (tirzepatide) injection, for subcutaneous use, contains tirzepatide, a GIP receptor and GLP-1 receptor agonist. Tirzepatide is based on the GIP sequence and contains 2 non-coded amino acids (aminoisobutyric acid, Aib) in positions 2 and 13, a C-terminal amide, and Lys residue at position 20 that is attached to 1,20-eicosanedioic acid via a linker. The molecular weight is 4813.53 Da and the empirical formula is C225H348N48O68.
Structural formula:
ZEPBOUND is a clear, colorless to slightly yellow, sterile, preservative-free solution for subcutaneous use. Each single-dose pen contains 0.5 mL solution of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg of tirzepatide and the following excipients: sodium chloride (4.1 mg), sodium phosphate dibasic heptahydrate (0.7 mg), and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH. ZEPBOUND has a pH of 6.5-7.5.
| Dosage Forms and Strengths |
|---|
|
Injection: Clear, colorless to slightly yellow solution available in pre-filled single-dose pens of the following strengths:
|
| How Supplied | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ZEPBOUND is a clear, colorless to slightly yellow solution available in pre-filled single-dose pens as follows:
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ZEPBOUND | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.