ZELSUVMI Topical gel Ref.[107468] Active ingredients: Berdazimer
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
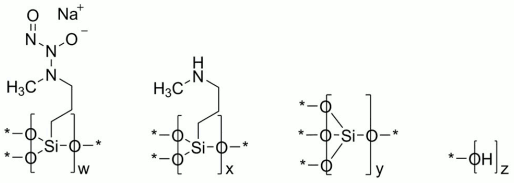
ZELSUVMI (berdazimer) topical gel, 10.3%, a nitric oxide releasing agent, contains the drug substance berdazimer sodium, a white to off white powder with the chemical name poly[{[3-(methylamino)propyl]silasesquioxane}co{[3-(1-methyl-2-nitroso2-oxidohydrazin-1-yl)propyl]silasesquioxane}-co-silicate (1:3:6 x)], partially hydrolyzed (Si:OH ~10:5), and the following structural and empirical formula:
Structural Formula:
* Denotes shared oxygen atom between bonded constituents; resulting bonds are Si-O-Si or Si‑OH
Empirical formula: [(C4H9N3NaO3.5Si)3(C4H10NO1.5Si)1(SiO2)6(HO0.5)5]0.1n
Due to the insoluble nature of berdazimer sodium, the molecular formula, molecular mass, and average molecular weight range cannot be determined.
ZELSUVMI (berdazimer) topical gel is an opaque white to off-white gel containing 10.3% berdazimer (equivalent to 10.9% berdazimer sodium). ZELSUVMI is supplied as two gel components that are mixed before administration:
- Tube A (14 g): an opaque white to off-white gel containing 240 mg of berdazimer sodium per gram of gel and the inactive ingredients cyclomethicone, hexylene glycol, hydroxypropyl cellulose, and isopropyl alcohol.
- Tube B (17 g): a translucent to opaque white to off-white gel containing the inactive ingredients benzoic acid, carboxymethylcellulose sodium, cyclomethicone, ethanol (13% v/v), glycerin, potassium phosphate monobasic, and purified water.
| Dosage Forms and Strengths |
|---|
|
Topical gel: 10.3% berdazimer in an opaque white to off-white gel. ZELSUVMI is supplied as two tubes. Tube A with a blue label contains 14 grams of berdazimer gel and Tube B with a yellow label contains 17 grams of hydrogel. |
| How Supplied |
|---|
|
ZELSUVMI (berdazimer) topical gel, 10.3% is supplied in a carton (NDC 71403-103-31) containing:
Manufactured for: EPIH SPV, LLC, Wilmington, Delaware 19801 |
Drugs
| Drug | Countries | |
|---|---|---|
| ZELSUVMI | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.