ZEMCELPRO Dispersion for infusion Ref.[116219] Active ingredients:
Source: European Medicines Agency (EU) Revision Year: 2026 Publisher: Cordex Biologics International Limited, 5 th Floor, Block E, Iveagh Court, Harcourt Road, Dublin, Ireland, Tel: 353 1 960 2797, e-mail: info@cordexbio.com
4.1. Therapeutic indications
Zemcelpro is indicated for the treatment of adult patients with haematological malignancies requiring an allogeneic haematopoietic stem cell transplantation following myeloablative conditioning for whom no other type of suitable donor cells is available.
4.2. Posology and method of administration
Zemcelpro must be administered in a qualified transplant centre with expertise in haematopoietic stem cell transplant by a physician with experience in the treatment of haematologic malignancies.
Posology
Treatment consists of a single dose for infusion containing a dispersion for infusion of expanded CD34+ cells in 1 to 4 infusion bags and unexpanded CD34- cells in 4 infusion bags.
The target dose is 0.4 to 7.5×106 viable CD34+ cells/kg for the expanded CD34+ cell component (dorocubicel) and ≥0.52×106 viable CD3+ cells/kg for the unexpanded CD34- cell component.
See the accompanying Release for infusion certificate (RfIC) for additional information pertaining to dose.
Cord blood unit selection
Erythrodepleted cord blood unit (CBU) for expansion is to be selected by the prescriber while respecting minimal requirement for human leukocyte antigen (HLA) matching and cell dose (i.e. pre-freeze CD34 cell count ≥0.5×105/kg and total nucleated cell (TNC) ≥1.5×107/kg). Matching for at least 4 of 6 HLA (HLA-A antigens, HLA-B antigens, and HLA-DRB1 alleles) is recommended, with a target 6 out of 8 HLA-match (high resolution typing). The HLA typing and nucleated cell content for each individual CBU used as starting material in the manufacture of Zemcelpro are provided in the accompanying RfIC.
Pre-treatment myeloablative conditioning (lymphodepleting chemotherapy)
An appropriate myeloablative conditioning regimen must be administered according to institutional guidelines. Selected regimen should be of high or intermediate intensity, i.e., with a transplant conditioning intensity (TCI) score of 2.5 and above. The conditioning regimen must not be initiated before ensuring that the availability of the patient-specific Zemcelpro is confirmed at the transplant centre.
The incorporation of anti-thymocyte globulin (ATG) is not recommended as part of the conditioning regimen (see section 4.5).
Prophylactic and supportive therapy for prevention of transplant complications
Prophylactic and supportive therapies for prevention of transplant complications (e.g., Graft-versus-Host Disease (GvHD), infection) must be administered according to institutional guidelines. Tacrolimus and mycophenolate mofetil combination is the preferred GvHD prophylaxis.
In the immediate post-transplant period, administration of granulocyte colony-stimulating factor (G-CSF) is recommended to minimize the risk of neutropenia and infection (see section 4.4).
Pre-medication
It is recommended that pre-medication with antipyretics, histamine antagonists, and antiemetics, according to local institutional guidelines, be administered 30-60 minutes before the infusion of both fractions of Zemcelpro to reduce the possibility of an infusion reaction. Moreover, pre-medication with corticosteroids is also recommended prior to administering the unexpanded CD34- component of Zemcelpro to reduce the possibility of an infusion reaction in case of major HLA histocompatibility.
Special populations
Elderly
The safety and efficacy of Zemcelpro in the elderly population (aged ≥65 years or older) have not been established.
Renal impairment
Zemcelpro has not been studied in patients with renal impairment. Patients should be assessed for renal impairment to determine transplant eligibility.
Hepatic impairment
Zemcelpro has not been studied in patients with hepatic impairment. Patients should be assessed for hepatic impairment to determine transplant eligibility.
Paediatric population
The safety and efficacy of Zemcelpro in children and adolescents below 18 years of age have not yet been established. Currently available data are described in section 5.1.
Method of administration
Prescribed number of bags of dorocubicel (1 to 4 bags) and of unexpanded CD34- cells (always 4 bags) must be infused to complete a single dose of Zemcelpro. The total number of infusion bags to be administered must be confirmed with the patient specific information on the RfIC.
Dorocubicel is infused first, followed by the unexpanded CD34- cells. It is recommended that the unexpanded CD34- cells be infused the same day as dorocubicel, but no later than the following day.
If dorocubicel is not administered, the unexpanded CD34- cells must not be infused to avoid any untoward immune reaction.
In case of infusion reaction, pausing infusion and instituting supportive care is recommended, as needed (see section 4.4).
Do not dilute, wash or sample Zemcelpro prior to infusion.
Intravenous use only. Central venous access is recommended for the infusion of Zemcelpro.
- Prepare infusion material. A latex-free tubing with a standard infusion filter (170-260 μm) must be used. Do NOT use a leukocyte depleting filter.
- Confirm i) the patient's identity with the patient identifiers on the bag and ii) the cell component identity (dorocubicel or unexpanded CD34- cells).
- Remove the overwrap and inspect the content of the thawed infusion bag for any visible cell aggregates. If visible cell aggregates are present, gently mix the contents of the bag, small aggregates of cellular material should disperse with gentle manual mixing. Remaining aggregates are effectively removed through filtration before infusion.
- The thawed and inspected bag must be infused promptly at approximately 10 to 20 mL per minute by gravity flow. Zemcelpro is stable between 15°C-30°C for up to 1 hour after end of thawing.
- Prime the tubing prior to infusion with sodium chloride 9 mg/mL (0.9%) solution for injection.
- Infuse all contents of the infusion bag (20 mL per bag).
- Rinse twice the infusion bag with 10 mL to 30 mL sodium chloride 9 mg/mL (0.9%) solution for injection by back priming to ensure the totality of cells are infused into the patient.
- The procedure for infusion must be repeated for the other bags. Wait to thaw and infuse the next bag until it is determined that the previous bag is safely administered.
Do not infuse Zemcelpro if the infusion bag is damaged or leaking or otherwise appears to be compromised.
4.9. Overdose
Risk of overdose is limited. There were no cases of overdose during clinical trials.
All 4 bags of the unexpanded CD34- cells component resulting from the manufacturing of Zemcelpro will always be infused. In very rare circumstances only, the release for infusion certificate (RfIC) will dictate not to administer all 4 bags of the expanded CD34+ cells component (dorocubicel) resulting from the manufacturing of Zemcelpro. Should this not be strictly followed, the resulting overdose of dorocubicel could be associated with an increased risk of infusion reactions and engraftment syndrome. Patients will need to be monitored for the occurrence of such events.
6.3. Shelf life
Cryopreserved: 1 year.
Once thawed: 1 hour at 15°C – 30°C.
Do not refreeze thawed medicinal product.
6.4. Special precautions for storage
Zemcelpro must be stored and transported in the vapour phase of liquid nitrogen (≤ −150°C) and must remain frozen until the patient is ready for treatment to ensure viable cells are available for patient administration.
For storage conditions after thawing of the medicinal product, see section 6.3.
6.5. Nature and contents of container and special equipment for use, administration or implantation
Zemcelpro is packaged in an ethylene vinyl acetate (EVA) infusion bag (50 mL) two ports containing 20 mL cell dispersion.
Each infusion bag is placed into an overwrap. This secondary packaging layer made of EVO copolymer is sealed twice. Each infusion bag in sealed overwrap is placed into a metallic cassette. The cassettes are subsequently placed in a labelled modpak within a standard, controlled cryoshipper.
One individual treatment dose comprises up to eight (8) infusion bags of 20 mL each, up to four (4) bags of dorocubicel, and four (4) bags of unexpanded CD34- cells.
Also provided with Zemcelpro is a cryovial containing the sample of the original cord blood unit for chimerism monitoring.
6.6. Special precautions for disposal and other handling
Precautions to be taken before handling or administering the medicinal product
Zemcelpro must be transported within the facility in closed, break-proof, leak-proof containers.
Zemcelpro must be transported in a container maintaining the product below -150°C and should be handled with appropriate protective gowning and gloves.
This medicinal product contains human blood cells. Healthcare professionals handling Zemcelpro must take appropriate precautions (wearing gloves, protective clothing and eye protection) to avoid potential transmission of infectious diseases.
Preparation prior to administration
Zemcelpro is composed of two (2) allogeneic hematopoietic cell components:
- Dorocubicel (expanded CD34+ cells)
- Unexpanded CD34- cells
Confirmation of the number of dorocubicel bags (1 to 4 bags) and the number of bags for unexpanded CD34- cells (always 4 bags) to be infused must be done based on release for infusion certificate (RfIC) prescription. The RfIC includes both components.
Dorocubicel is infused first, followed by the unexpanded CD34- cells. It is recommended that the unexpanded CD34- cells be infused the same day as dorocubicel, but no later than the following day.
Coordinate the timing of Zemcelpro thaw and infusion in the following manner: confirm the readiness of the patient to be infused in advance and adjust the start time of Zemcelpro thaw such that it will be available for infusion when the patient is ready.
Thawing
Prior to Zemcelpro thawing, confirm patient's identity and numbers of bags to infused according to the infusion certificate (RfIC). Thaw all prescribed bags of dorocubicel before bags from unexpanded CD34- cells. Thaw one (1) bag at a time. Wait to thaw the next bag until it is determined that the previous bag is safely administered.
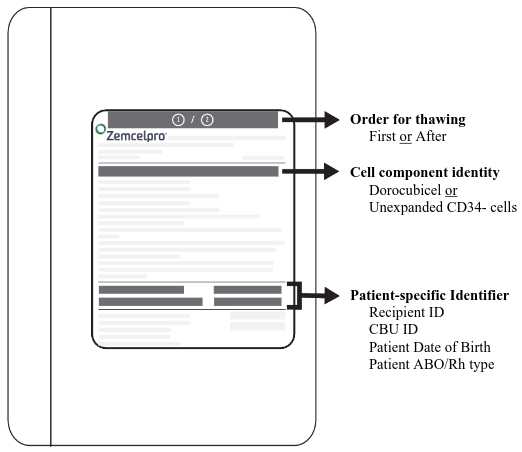
Figure 1. Zemcelpro Storage Cassette:
- Retrieve the storage cassette from the cryoshipper. Confirm i) the patient's identity with the patient identifiers on the cassette and ii) the cell component identity (dorocubicel or unexpanded CD34- cells) (Figure 1).
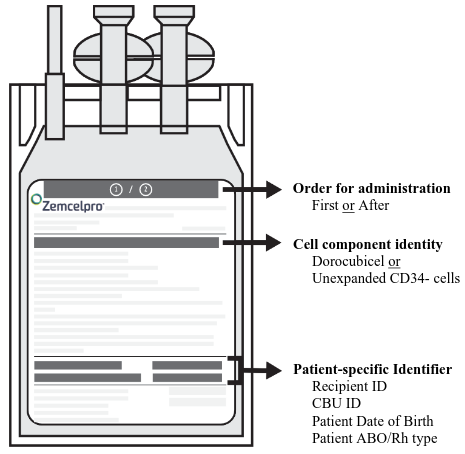
- After cassette verification, immediately remove the infusion bag from the cassette. Confirm i) the patient's identity with the patient identifiers on the infusion bag and ii) the cell component identity (dorocubicel or unexpanded CD34- cells) (Figure 2).
- Inspect the infusion bag(s) for any breaks or cracks prior to thawing. If a bag is compromised, do not infuse the contents.
- Place immediately the infusion bag contained in its sealed overwrap in a 37°C water bath. When semi-liquid consistency is reached, start to gently knead the bag until no crystal ice remains. The complete thawing duration takes approximately 2-5 minutes per bag.
- Remove bag with overwrap from the water bath. Once the infusion bag has been thawed, it should be infused as promptly as possible. Zemcelpro has been shown to be stable between 15°C – 30°C for up to 1 hour. Do not dilute, wash, or sample Zemcelpro prior to infusion.
- Unless prepared at the patient's bedside, transport the product to the bedside at room temperature in a closed box/bag to protect the product during transport. Do not infuse Zemcelpro if the infusion bag is damaged or leaking or otherwise appears to be compromised.
Administration
Prescribed number of bags of dorocubicel (1 to 4 bags) and of unexpanded CD34- cells (always 4 bags) must be infused to complete a single dose of Zemcelpro. The total number of infusion bags to be administered must be confirmed with the patient specific information on the RfIC.
Dorocubicel is infused first, followed by the unexpanded CD34- cells. It is recommended that the unexpanded CD34- cells be infused the same day as dorocubicel, but no later than the following day.
If dorocubicel is not administered, the unexpanded CD34- cells must not be infused to avoid any untoward immune reaction.
In case of infusion reaction, pausing infusion and instituting supportive care is recommended, as needed (see section 4.4).
Do not dilute, wash or sample Zemcelpro prior to infusion.
Intravenous use only. Central venous access is recommended for the infusion of Zemcelpro.
Figure 2. Zemcelpro Infusion Bag:
- Prepare infusion material. A latex-free tubing with a standard infusion filter (170-260 μm) must be used. Do NOT use a leukocyte depleting filter.
- Confirm i) the patient's identity with the patient identifiers on the bag and ii) the cell component identity (dorocubicel or unexpanded CD34- cells) (Figure 2).
- Remove the overwrap and inspect the content of the thawed infusion bag for any visible cell aggregates. If visible cell aggregates are present, gently mix the contents of the bag, small aggregates of cellular material should disperse with gentle manual mixing. Remaining aggregates are effectively removed through filtration before infusion.
- The thawed and inspected bag must be infused promptly at approximately 10 to 20 mL per minute by gravity flow. Zemcelpro is stable between 15°C – 30°C for up to 1 hour after end of thawing.
- Prime the tubing prior to infusion with sodium chloride 9 mg/mL (0.9%) solution for injection.
- Infuse all contents of the infusion bag (20 mL per bag).
- Rinse twice the infusion bag with 10 mL to 30 mL sodium chloride 9 mg/mL (0.9%) solution for injection by back priming to ensure the totality of cells are infused into the patient.
- The procedure for infusion must be repeated for the other bags. Wait to infuse the next bag until it is determined that the previous bag is safely administered.
Do not infuse Zemcelpro if the infusion bag is damaged or leaking or otherwise appears to be compromised.
Measures to take in case of accidental exposure
Follow local guidelines on handling of human-derived material in case of accidental exposure. Work surfaces and materials which have potentially been in contact with Zemcelpro must be decontaminated with appropriate disinfectant.
Precautions to be taken for the disposal of the medicinal product
Unused medicinal product and all material that has been in contact with Zemcelpro (solid and liquid waste) must be handled and disposed of as potentially infectious waste in accordance with local guidelines on handling of human derived material.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.