ZEPZELCA Powder for solution for injection Ref.[50657] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
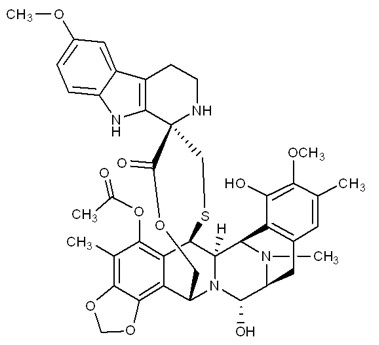
ZEPZELCA is an alkylating drug. The chemical name of ZEPZELCA (lurbinectedin) is (1’R,6R,6aR,7R,13S,14S,16R)-8,14-dihydroxy-6',9-dimethoxy-4,10,23-trimethyl-19-oxo-2',3',4',6,7,9',12,13,14,16-decahydro-6aH-spiro[7,13-azano-6,16-(epithiopropanooxymethano) [1,3]dioxolo[7,8]isoquinolino[3,2-b]3benzazocine-20,1'-pyrido[3,4-b]indol]-5-yl acetate.
The molecular formula is C41H44N4O10S. The molecular weight is 784.87g/mol, and the chemical structure is:
ZEPZELCA for injection 4 mg is supplied as a lyophilized powder in a single-dose vial for reconstitution for intravenous use. The ZEPZELCA lyophilized formulation is comprised of 4 mg lurbinectedin, sucrose (800 mg), lactic acid (22.1 mg), and sodium hydroxide (5.1 mg). Before use, the lyophilizate is reconstituted by addition of 8 mL Sterile Water for Injection USP, yielding a solution containing 0.5 mg/mL lurbinectedin (the calculated concentration is 0.47 mg/mL based on the final volume of 8.5 mL).
| Dosage Forms and Strengths |
|---|
|
For injection: 4 mg of lurbinectedin as a sterile, preservative-free, white to off-white lyophilized powder in a single-dose vial for reconstitution prior to intravenous infusion. |
| How Supplied |
|---|
|
ZEPZELCA (lurbinectedin) for injection is supplied as a sterile, preservative-free, white to off‑white lyophilized powder in a single-dose clear glass vial. Each carton (NDC 68727‑712-01) contains 4 mg in one single-dose vial. Distributed by: Jazz Pharmaceuticals, Inc., Palo Alto, CA 94304 |
Drugs
| Drug | Countries | |
|---|---|---|
| ZEPZELCA | Ecuador, Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.