ZIENT Tablet Ref.[50664] Active ingredients: Ezetimibe
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2021 Publisher: Organon Pharma Pty Limited, Building A, 26 Talavera Road, Macquarie Park NSW 2113
Product name and form
ZIENT.
Ezetimibe.
| Pharmaceutical Form |
|---|
|
ZIENT (ezetimibe): 10 mg, white to off-white capsule shaped tablets, debossed with “414” on one side. |
Qualitative and quantitative composition
Each tablet of ZIENT for oral administration contains 10 mg ezetimibe.
List of excipients with known effect: lactose (as monohydrate).
For the full list of excipients, see Section 6.1 List of Excipients.
Physicochemical properties
Ezetimibe is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Ezetimibe has a melting point of about 163°C and is stable at ambient temperature.
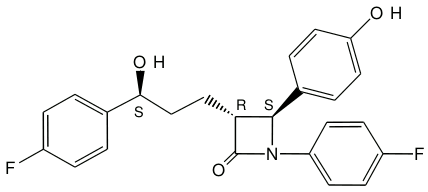
ZIENT, ezetimibe is described chemically as 1-(4-fluorophenyl)3==®==[3-(4-fluorophenyl)-3(S)-hydroxypropyl]4(S)(4-hydroxyphenyl)-2-azetidinone.
The empirical formula is C24H21F2NO3.
Its molecular weight is 409.4.
Chemical structure:
Its structural formula is:
CAS number:
The CAS registry number is 163222-33-1.
| Active Ingredient |
|---|
|
Ezetimibe is in a new class of lipid-lowering compounds that selectively inhibit the intestinal absorption of cholesterol. The molecular target of ezetimibe is the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is responsible for the intestinal uptake of cholesterol. |
| List of Excipients |
|---|
|
Each 10 mg tablet contains croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium lauryl sulfate. |
Pack sizes and marketing
Supplied in blister packs of 5, 10 and 30.
Marketing authorization holder
Organon Pharma Pty Limited, Building A, 26 Talavera Road, Macquarie Park NSW 2113
Marketing authorization dates and numbers
Date of first approval: 22 December 2015
Drugs
| Drug | Countries | |
|---|---|---|
| ZIENT | Australia, Ecuador, Mexico, Singapore |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.