ZILBRYSQ Solution for injection Ref.[51701] Active ingredients: Zilucoplan
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: UCB Pharma S.A., Allée de la Recherche 60, B-1070 Bruxelles, Belgium
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, complement inhibitors
ATC code: L04AJ06
Mechanism of action
Zilucoplan is a 15 amino acid, synthetic macrocyclic peptide that inhibits the effects of the complement protein C5 through a dual mechanism of action. It specifically binds to C5, thereby inhibiting its cleavage by the C5 convertase to C5a and C5b, which results in a downregulation of the assembly and cytolytic activity of the membrane attack complex (MAC). Additionally, by binding to the C5b moiety of C5, zilucoplan sterically hinders binding of C5b to C6, which prevents the subsequent assembly and activity of the MAC, should any C5b be formed.
Pharmacodynamic effects
The pharmacodynamic effect of zilucoplan was analysed through the ability of inhibiting ex vivo, complement-induced sheep red blood cell (sRBC) lysis.
Data from the phase 2 and phase 3 studies demonstrate rapid, complete (>95%) and sustained complement inhibition with zilucoplan when dosed according to Table 1.
Clinical efficacy and safety
The safety and efficacy of zilucoplan were evaluated in a 12-week multicentre, randomised, double-blind placebo-controlled study MG0010 (RAISE) and the open-label extension study MG0011 (RAISE-XT).
Study MG0010 (RAISE)
A total of 174 patients were enrolled, who were at least 18 years of age, had acetylcholine-receptor antibody positive generalised myasthenia gravis, a Myasthenia Gravis- Activities of Daily Living (MG-ADL) Score of ≥ 6 and a Quantitative Myasthenia Gravis (QMG Score) of ≥ 12 (see Table 3).
Patients were treated once daily with either zilucoplan (dosed according to Table 1) or placebo with 86 and 88 patients randomised to each treatment group, respectively. Stable standard of care (SOC) therapy was allowed. The majority of patients received treatment for gMG at baseline which included parasympathomimetics (84.5%), systemic corticosteroids (63.2%) and nonsteroidal immunosuppressants (51.1%).
The primary endpoint was the change from baseline to week 12 in MG-ADL total score. Key secondary endpoints were the change from baseline to week 12 in QMG total score, in Myasthenia Gravis Composite (MGC) total score and in MG Quality of Life (MG-QoL15r) total score (Table 4).
MG-ADL clinical responders were defined as having at least a 3-point decrease and QMG responders were defined as having at least a 5-point decrease without rescue therapy.
Table 3. Baseline demographic and disease characteristics of patients enrolled in study MG0010:
| Zilucoplan (n=86) | Placebo (n=88) | |
|---|---|---|
| Age, years, mean (SD) | 52.6 (14.6) | 53.3 (15.7) |
| Age at onset, years, mean (SD) | 43.5 (17.4) | 44.0 (18.7) |
| Age ≥65 | 22 (25.6) | 26 (29.5) |
| Gender, male, n (%) | 34 (39.5) | 41 (46.6) |
| Baseline MG-ADL score mean (SD) | 10.3 (2.5) | 10.9 (3.4) |
| Baseline QMG score mean (SD) | 18.7 (3.6) | 19.4 (4.5) |
| Baseline MGC score, mean (SD) | 20.1 (6.0) | 21.6 (7.2) |
| Baseline MG-QoL 15r score, mean (SD) | 18.6 (6.6) | 18.9 (6.8) |
| Duration of disease, years, mean (SD) | 9.3 (9.5) | 9.0 (10.4) |
| MGFA class at screening, n (%) Class II | 22 (25.6) | 27 (30.7) |
| MGFA class at screening, n (%) Class III | 60 (69.8) | 57 (64.8) |
| MGFA class at screening, n (%) Class IV | 4 (4.7) | 4 (4.5) |
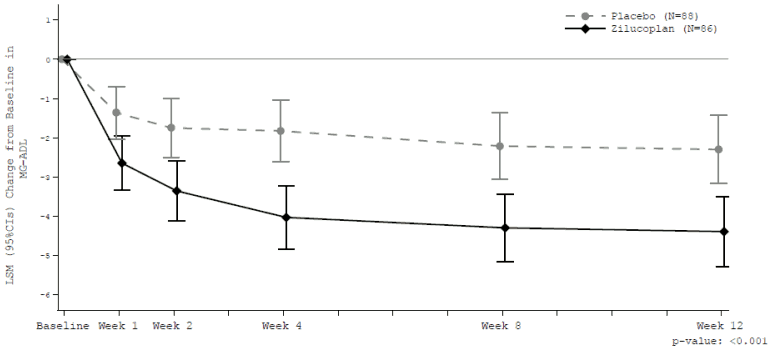
Table 4 presents the change from baseline at week 12 in the total scores for MG-ADL, QMG, MGC and MGQoL15r. Mean baseline scores were 10.9 and 10.3 for MG-ADL, 19.4 and 18.7 for QMG, 21.6 and 20.1 for MGC and 18.9 and 18.6 for MG-QoL15r for placebo and zilucoplan groups, respectively.
Table 4. Change from baseline at week 12 in total scores for MG-ADL, QMG, MGC and MG-QoL15r:
| Endpoints: Change from baseline in total score at week 12: LS Mean (95% CI) | Zilucoplan (n=86) | Placebo (n=88) | Zilucoplan change LS mean difference vs. placebo (95% CI) | p-value* |
|---|---|---|---|---|
| MG-ADL | -4.39 (-5.28, -3.50) | -2.30 (-3.17, -1.43) | -2.09 (-3.24, -0.95) | <0.001 |
| QMG | -6.19 (-7.29, -5.08) | -3.25 (-4.32, -2.17) | -2.94 (-4.39, -1.49) | <0.001 |
| MGC | -8.62 (-10.22, -7.01) | -5.42 (-6.98, -3.86) | -3.20 (-5.24, -1.16) | 0.0023 |
| MG-QoL15r | -5.65 (-7.17, -4.12) | -3.16 (-4.65, -1.67) | -2.49 (-4.45, -0.54) | 0.0128 |
* Analysis based on a MMRM ANCOVA model
The treatment effect in the zilucoplan group for all 4 endpoints started rapidly at week 1, further increased to week 4 and was sustained through week 12.
At week 12, a clinically meaningful and highly statistically significant improvement in MG-ADL total score (Figure 1) and in QMG total score was observed for zilucoplan versus placebo.
Figure 1. Change from baseline in MG ADL total score:
Analysis based on MMRM ANCOVA model
Clinically meaningful change = 2-point change in MG-ADL score
At week 12, 73.1% of the patients in the zilucoplan group were MG-ADL clinical responders without rescue therapy, vs. 46.1% in the placebo group (p<0.001). Fifty-eight percent (58.0%) of the patients in the zilucoplan group were QMG clinical responders without rescue therapy, vs. 33.0% in the placebo group (p=0.0012).
At week 12, the cumulative portion of patients that needed rescue therapy was 5% in the zilucoplan group and 11% in the placebo group. Rescue therapy was defined as intravenous immunoglobulin G (IVIG) or plasma exchange (PLEX).
Study MG0011 (RAISE-XT)
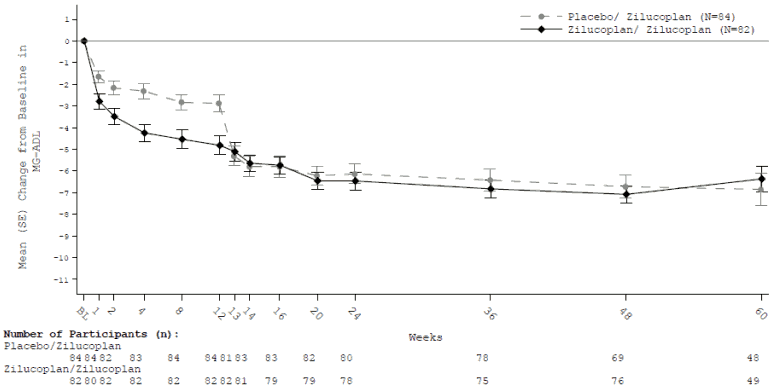
Two hundred patients who completed a placebo-controlled phase 2 study (MG0009) or the phase 3 study (MG0010) continued in the open-label extension study MG0011 in which all patients received zilucoplan (dosed according to Table 1) daily. Primary objective was long-term safety. Secondary efficacy endpoints were change from double-blind study baseline in MG-ADL, QMG, MGC and MG-QoL15r score at week 24. For former MG0010 participants, results are shown below (Table 5).
Table 5. Mean change from double-blind study baseline (MG0010) to week 24 (week 12 in MG0011) and week 60 (week 48 in MG0011) in total scores for MG-ADL, QMG, MGC and MG-QoL15r:
| Endpoints: Change from baseline in total score at week 24 and week 60: LS Mean (95% CI) | Zilucoplan (n=82) | Placebo/zilucoplan (n=84) |
|---|---|---|
| MG-ADL | ||
| Week 24 | -5.46 (0.59) | -5.20 (0.52) |
| Week 60 | -5.16 (0.61) | -4.37 (0.54) |
| QMG | ||
| Week 24 | -7.10 (0.80) | -7.19 (0.69) |
| Week 60 | -6.44 (0.83) | -6.15 (0.71) |
| MGC | ||
| Week 24 | -10.37 (1.15) | -11.12 (1.00) |
| Week 60 | -8.89 (1.20) | -9.01 (1.04) |
| MG-QoL15r | ||
| Week 24 | -8.09 (0.96) | -7.96 (0.89) |
| Week 60 | -7.22 (0.99) | -6.09 (0.91) |
Analysis based on a MMRM ANCOVA model where rescue therapy and discontinuation are imputed as treatment failure; Death are imputed the worst possible score (e.g. score 24 for MG-ADL).
SE = Standard error
Figure 2. Mean change from double-blind study baseline to week 60 for total MG ADL score:
Immunogenicity
In MG0010 and MG0011 (RAISE-XT), the patients were tested for anti-drug antibody (ADA) positivity and anti-polyethylene glycol (PEG) antibody positivity.
In both studies, antibody titres were low and there was no evidence of an impact on pharmacokinetics or pharmacodynamics and no clinically meaningful impact on efficacy or safety.
In MG0010 and MG0011, 2 patients (2.4%) each in the zilucoplan/zilucoplan and placebo/zilucoplan group were positive for treatment emergent ADA and anti-PEG antibodies. Thirteen subjects (16%) per arm were treatment emergent anti-PEG antibody positive while ADA negative. Two patients (2.4%) per arm were anti-PEG negative while treatment emergent ADA positive.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with zilucoplan in one or more subsets of the paediatric population in the treatment of myasthenia gravis. See section 4.2 for information on paediatric use.
5.2. Pharmacokinetic properties
Absorption
Following single and multiple daily subcutaneous administration of the zilucoplan recommended dose (Table 1) in healthy subjects, zilucoplan reached peak plasma concentration generally between 3 to 6 hours post-dose.
In study MG0010 in patients with gMG, after daily repeated subcutaneous administration of the zilucoplan recommended dose (Table 1), plasma concentrations of zilucoplan were consistent, with steady state trough concentrations being reached by week 4 and maintained through week 12. Exposures after subcutaneous administration of single zilucoplan doses in the abdomen, thigh, or upper arm were comparable.
Distribution
Zilucoplan and the active (RA103488) and major inactive (RA102758) circulating metabolites are highly bound to plasma proteins (>99%). The mean volume of distribution for zilucoplan (Vc/F) using a population pharmacokinetic analysis is 3.51 L. Zilucoplan is not a substrate for common drug transporters.
Metabolism
Zilucoplan is not a substrate of major CYP enzymes. In plasma, 2 metabolites, the active (RA103488) and major inactive metabolite (RA102758) were detected. The formation of RA103488 is mainly due to cytochrome CYP450 4F2. RA103488 has pharmacological activity similar to zilucoplan but is present at a much lower concentration compared to zilucoplan. The contribution of RA103488 to pharmacological activity is low. Further, as a peptide, zilucoplan is expected to be degraded into smaller peptides and amino acids via catabolic pathways.
Zilucoplan inhibits MRP3 in vitro at therapeutic concentrations; the clinical relevance of this inhibition is unknown.
Elimination
As a peptide, zilucoplan is expected to be degraded into smaller peptides and amino acids via catabolic pathways. The mean plasma terminal elimination half-life was approximately 172 hours (7-8 days). The half-life was 220 hours and 96 hours respectively for the active (RA103488) and major inactive metabolite (RA102758). The excretion of zilucoplan and its metabolites (RA103488 and RA102758) measured in both urine and faeces was negligible. The pegylated part of zilucoplan is anticipated to be excreted mainly via the kidneys and the main degradation of fatty acid part is via β-oxidation to acetyl-CoA.
Linearity/non-linearity
In the population pharmacokinetic analysis (doses corresponding to 0.05 to 0.6 mg/kg), zilucoplan pharmacokinetics is characterised by target dependent drug disposition with less than dose proportional increase in exposure with increasing doses, and after multiple doses compared to single dose.
Antibodies
The incidences of ADA and anti-PEG antibodies in the phase 3 study in patients with gMG were comparable between the zilucoplan treatment group and the placebo treatment group (see section 5.1). The ADA and anti-PEG antibody status of patients treated with zilucoplan did not affect zilucoplan concentrations.
Special populations
Weight
Population pharmacokinetic analysis on data collected across studies in gMG showed that body weight significantly influences the pharmacokinetics of zilucoplan. Zilucoplan dosing is based on body weight categories (see section 4.2), no further dose adjustment is needed.
Elderly
Based on population pharmacokinetic analysis, age did not influence the pharmacokinetics of zilucoplan. No dose adjustment is required.
Renal impairment
The effect of renal impairment on the pharmacokinetics of zilucoplan and its metabolites was studied in an open-label phase 1 study, where a single-dose of the zilucoplan recommended dose (Table 1) was administered to healthy subjects and subjects with severe renal impairment (creatinine clearance between 15 and <30 mL/min).
Systemic exposure to zilucoplan and the major inactive metabolite RA102758 was not different in subjects with severe renal impairment compared to subjects with normal renal function. The exposure to the active metabolite RA103488 was approximately 1.5-fold higher in subjects with severe renal impairment compared to subjects with normal renal function.
Based on the pharmacokinetic results, no dose adjustment is required in patients with renal impairment.
Hepatic impairment
The effects of moderate hepatic impairment (as defined by a Child-Pugh score between 7 and 9) on the pharmacokinetics of zilucoplan and its metabolites were studied in an open-label phase 1 study, where a single dose of the zilucoplan recommended dose (Table 1) was administered to healthy subjects and subjects with moderate hepatic impairment.
Systemic exposure to zilucoplan was 24% lower in subjects with moderate impaired liver function compared to healthy subjects, which was in line with a higher systemic and peak exposures of both metabolites in subjects with hepatic impairment compared to healthy subjects. Zilucoplan peak exposure as well as terminal half-life were comparable between both groups. Further pharmacodynamic analysis did not identify meaningful differences in complement levels or inhibition of complement activity between both groups. Based on these results, no dose adjustment is required in patients with mild and moderate hepatic impairment.
Racial and ethnic groups
In a phase 1 clinical study in healthy Caucasian and Japanese subjects, the pharmacokinetic profile of zilucoplan and its two metabolites (RA102758 and RA103488) was compared following a single dose (Table 1) and after multiple dosing for 14 days. Results were generally similar between both groups. The population pharmacokinetic analysis for zilucoplan showed that there are no differences between the different race categories (Black/African American, Asian/Japanese, and Caucasians). No dose adjustment is required.
Gender
In the population pharmacokinetic analysis, no difference in pharmacokinetics between genders was observed. No dose adjustment is required.
5.3. Preclinical safety data
In repeat-dose toxicity studies performed in non-human primates, there were vesicular degeneration/hyperplasia of epithelial cells and mononuclear cell infiltrates in various tissues at clinically relevant exposure. In the pancreas, this sometimes manifested as pancreatic acinar cell degeneration, some with fibrosis and ductal degeneration/regeneration and was accompanied with increased plasma concentrations of amylase and lipase. In female reproductive organs (vagina, cervix, uterus), mononuclear cell infiltrates with epithelial degeneration and cervical squamous metaplasia were seen. In a monkey male fertility study, minimal to slight germ line degeneration/depletion was observed at clinically relevant exposures but severity did not increase with dose. No impact on spermatogenesis was observed.The findings in non-human primates are of uncertain clinical relevance and some are possibly related to infections secondary to the pharmacological effect of zilucoplan, but other mechanisms cannot be excluded. The findings did not correlate with any effects on embryofetal development or pregnancy outcomes (pregnancy loss, parturition, pregnancy outcomes, or infant postnatal development) in non-human primates at similar dose levels.
No carcinogenicity studies were conducted with zilucoplan.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.