ZINC SULFATE Solution for injection Ref.[51049] Active ingredients: Zinc sulfate
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
Zinc Sulfate Injection, USP is a sterile, clear, colorless solution, essentially free from visible particles intended for use as a trace element and an additive to intravenous solutions for parenteral nutrition.
10 mg per 10 mL Pharmacy Bulk Package vial: Each mL contains 1 mg of zinc present as a 2.46 mg of zinc sulfate and water for injection, q.s.
30 mg per 10 mL Pharmacy Bulk Package vial: Each mL contains 3 mg of zinc present as 7.41 mg of zinc sulfate and water for injection, q.s.
25 mg per 5 mL Pharmacy Bulk Package vial: Each mL contains 5 mg of zinc present as 12.32 mg of zinc sulfate and water for injection, q.s.
All presentations do not contain preservatives.
The pH range is 2 to 4; pH may be adjusted with sulfuric acid.
1 mg/mL of Zinc Sulfate Injection contains no more than 1,500 mcg/L of aluminum and has a calculated osmolarity of 33 mOsmol/L.
3 mg/mL of Zinc Sulfate Injection contains no more than 2,500 mcg/L of aluminum and has a calculated osmolarity of 96.5 mOsmol/L.
5 mg/mL of Zinc Sulfate Injection contains no more than 2,500 mcg/L of aluminum and has a calculated osmolarity of 157.2 mOsmol/L.
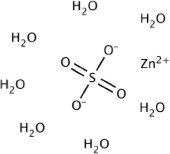
Zinc sulfate heptahydrate, USP has a molecular weight of 287.54 g/mol and a formula of ZnSO4·7H2O.
| Dosage Forms and Strengths |
|---|
|
Zinc Sulfate Injection, USP:
|
| How Supplied | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Zinc Sulfate Injection, USP is a sterile, clear, colorless solution, essentially free from visible particles supplied as:
The container closure is not made with natural rubber latex. FRESENIUS KABI, Lake Zurich, IL 60047 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.