ZINECARD Solution for injection Ref.[27445] Active ingredients: Dexrazoxane
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Proper name: Dexrazoxane
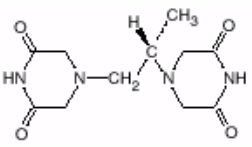
Chemical name: (S)4,4'(1-methyl-1,2-ethanediyl)bis[2,6-piperazinedione]
Molecular formula and molecular mass: C11H16N4O4 and 268.28
Structural formula:
Physicochemical properties: ZINECARD (dexrazoxane) is a sterile, lyophilized, parenteral, cardioprotective agent for use in conjunction with anthracycline. It is a white to off-white crystalline powder.
It is sparingly soluble in water and 0.1 N HCl, slightly soluble in acetonitrile, ethanol, methanol and water/dimethylacetamide (1:1), and practically insoluble in non-polar organic solvents. Its melting range is 187-197°C. The acidic pKa value of dexrazoxane at 25°C is 2.1. The alkaline pKa’s are 10.1 and 11.1. The partition coefficient, expressed as the dexrazoxane concentration in 1-octanol divided by the aqueous phase concentration, is 0.025 at 25°C.
| Dosage Forms and Strengths |
|---|
|
ZINECARD (dexrazoxane) is available in: 250 mg single dose vial, for reconstitution with recommended diluent. Diluent not provided. The 250 mg vial contains 250 mg of dexrazoxane; pH is adjusted with hydrochloric acid, NF. The 500 mg vial contains 500 mg of dexrazoxane; pH is adjusted with hydrochloric acid, NF. |
Drugs
| Drug | Countries | |
|---|---|---|
| ZINECARD | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.