ZOLGENSMA Solution for infusion Ref.[10923] Active ingredients: Onasemnogene abeparvovec
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Novartis Gene Therapies EU Limited, Block B, The Crescent Building, Northwood, Santry, Dublin 9, D09 C6X8, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other drugs for disorders of the musculo-skeletal system
ATC code: M09AX09
Mechanism of action
Onasemnogene abeparvovec is a gene therapy designed to introduce a functional copy of the survival motor neuron gene (SMN1) in the transduced cells to address the monogenic root cause of the disease. By providing an alternative source of SMN protein expression in motor neurons, it is expected to promote the survival and function of transduced motor neurons.
Onasemnogene abeparvovec is a non-replicating recombinant AAV vector that utilizes AAV9 capsid to deliver a stable, fully functional human SMN transgene. The ability of the AAV9 capsid to cross the blood brain barrier and transduce motor neurons has been demonstrated. The SMN1 gene present in onasemnogene abeparvovec is designed to reside as episomal DNA in the nucleus of transduced cells and is expected to be stably expressed for an extended period of time in post-mitotic cells. The AAV9 virus is not known to cause disease in humans. The transgene is introduced to target cells as a self-complementary double-stranded molecule. Expression of the transgene is driven by a constitutive promoter (cytomegalovirus enhanced chicken-β-actin-hybrid), which results in continuous and sustained SMN protein expression. Proof of the mechanism of action has been supported by nonclinical studies and by human biodistribution data.
Clinical efficacy and safety
AVXS-101-CL-303 Phase 3 study in patients with Type 1 SMA
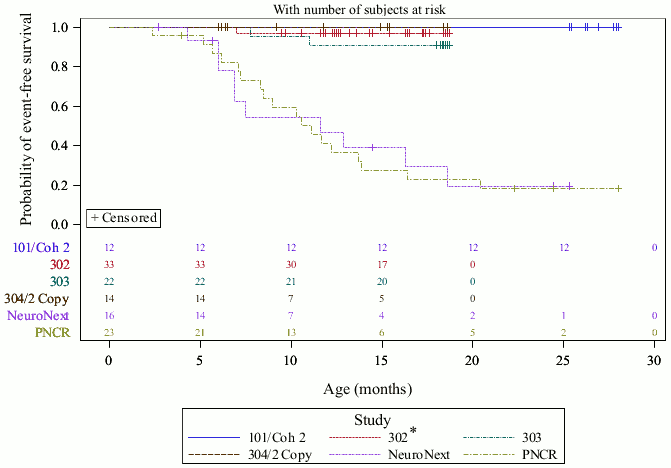
AVXS-101-CL-303 (Study CL-303) is a Phase 3 open-label, single-arm, single-dose study of intravenous administration of onasemnogene abeparvovec at the therapeutic dose (1.1 × 1014 vg/kg). Twenty-two patients were enrolled with Type 1 SMA and 2 copies of SMN2. Patient ages at administration ranged from 0.5 to 5.9 months. Of the 22 enrolled patients, 3 patients discontinued the study of which 2 patients had an event (death or permanent ventilation) leading to 90.9% (95% CI: 79.7%, 100.0%) event-free survival (alive without permanent ventilation) at 14 months of age, see Figure 1.
Figure 1. Time (months) to death or permanent ventilation pooled from onasemnogene abeparvovec IV studies (CL-101, CL-302, CL-303, CL-304-2 copy cohort):
PNCR = Pediatric Neuromuscular Clinical Research natural history cohort NeuroNext = Network for Excellence in Neuroscience Clinical Trials natural history cohort
* AVXS-101-CL-302 is an ongoing Phase 3 multicenter, open-label, single-arm, single-dose study of AVXS101 (gene replacement therapy) in patients with SMA Type 1 with 1 or 2 copies of the SMN2 gene similar to study AVXS-101-CL-303. The average age of the patients in the study at time of the 31 December 2019 data cutoff is 10.62 months (range: 1.8 to 15.4 months).
For the 14 patients in Study CL-303 that achieved the milestone of independent sitting for at least 30 seconds, the median age when this milestone was first demonstrated was 12.5 months (range: 9.2 to 18.6 months). Thirteen patients confirmed the milestone of independent sitting for at least 30 seconds at the 18-month visit (co-primary endpoint, p<0.0001). One patient achieved the milestone of sitting independently for 30 seconds at 16 months of age, but this milestone was not confirmed at the Month 18 visit. The video-confirmed developmental milestones for patients in Study CL-303 are summarised in Table 4. Three patients did not achieve any motor milestones (13.6%) and 6 patients (27.2%) achieved head control as the maximum motor milestone before the 18 months of age final study visit.
Table 4. Median time to video documented achievement of motor milestones Study 303:
| Video documented milestone | Number of patients achieving milestone n/N (%) | Median age to the milestone achievement (months) | 95% Confidence interval |
|---|---|---|---|
| Head control | 17/20* (85) | 6.8 | (4.77, 7.17) |
| Rolls from back to sides | 13/22 (59) | 11.5 | (7.77, 14.53) |
| Sits without support for 30 seconds (Bayley) | 14/22 (64) | 12.5 | (10.17, 15.20) |
| Sitting without support for at least 10 seconds (WHO) | 14/22 (64) | 13.9 | (11.00, 16.17) |
* 2 patients were reported to have Head Control by clinician assessment at baseline.
One patient (4.5%) could also walk with assistance at 12.9 months. Based on the natural history of the disease, patients who met the study entry criteria would not be expected to attain the ability to sit without support.
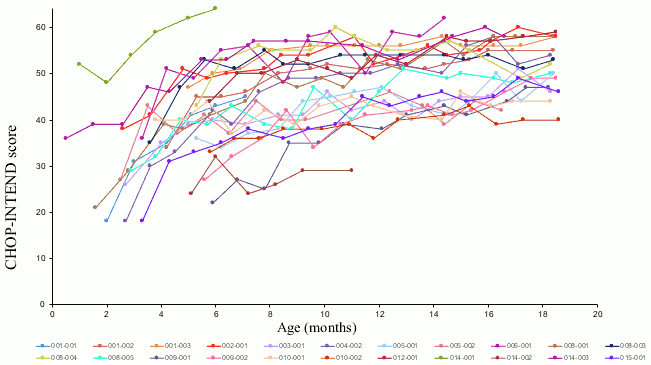
Motor function improvements were also observed as measured by the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND), see Figure 2. Twenty-one patients (95.5%) achieved a CHOP-INTEND score ≥40, 14 patients (64%) had achieved a CHOPINTEND score ≥50, and 5 patients (23%) had achieved a CHOP-INTEND score ≥60. Patients with untreated SMA Type 1 almost never achieve a CHOP-INTEND score ≥40. Motor milestone achievement was observed in some patients despite plateauing of CHOP-INTEND. No clear correlation was observed between CHOP-INTEND scores and motor milestone achievement.
Figure 2. CHOP-INTEND motor function scores - Study CL-303:
AVXS-101-CL-101 Phase 1 study in patients with Type 1 SMA
The results seen in Study 303 are supported by study AVXS-101-CL-101 (Phase 1 study in Type 1 SMA, Study CL-101) in which onasemnogene abeparvovec was administered as a single intravenous infusion in 12 patients from 2.6 kg to 8.5 kg (0.9 to 7.9 months of age). At 14 months of age, all treated patients were event-free; i.e. survived without permanent ventilation, compared to 25% in the natural history cohort. At the end of the study (24 months post-dose), all treated patients were eventfree, compared to less than 8% in the natural history, see Figure 1.
At 24 months of follow up post-dose, 10 out of 12 patients were able to sit without support for ≥10 seconds, 9 patients were able to sit without support for ≥30 seconds and 2 patients were able to stand and walk without assistance. One out of 12 patients did not achieve head control as the maximum motor milestone before the age of 24 months. Ten of 12 patients from Study CL-101 who received the proposed therapeutic dose of onasemnogene abeparvovec continue to be followed in a long-term study (for up to 5.7 years after dosing) and all have either maintained all previously attained milestones or even gained new milestones such as sitting with support, standing with assistance and walking alone. Four of the 10 patients received concomitant nusinersen treatment at some point during the long-term study. Maintenance of efficacy and achievement of milestones can therefore not be solely attributed to onasemnogene abeparvovec in all patients. The milestone of standing with assistance was newly acquired by 2 patients who were not receiving nusinersen.
AVXS-101-CL-304 Phase 3 study in patients with pre-symptomatic SMA
Study CL-304 is an ongoing, global, Phase 3, open-label, single-arm, single-dose, multicenter study of IV AVXS-101 in pre-symptomatic newborn patients up to 6 weeks of age with 2 (cohort 1, n=14) or 3 (cohort 2, n=15) copies of SMN2.
Cohort 1
At the time of the last study visit prior to 31 December 2019, treated patients with 2 copies of SMN2 were between 6 months and 18.6 months of age and had been in the study for an average of 10.5 months (range: 5.1 to 18.0 months). All patients were alive and free of permanent ventilation.
Eight patients achieved independent sitting for at least 30 seconds, at ages ranging from 6.4 to 11.8 months, with 7 of these 8 (87.5%) achieving independent sitting prior to the 9.2 months of age, the 99th percentile for development of this milestone. Four patients achieved the milestone of walking alone (28.6%). Twelve patients (85.7%) have achieved CHOP-INTEND scores ≥60 as of the 31 December 2019 data cut-off.
Cohort 2
At the time of the last study visit prior to 31 December 2019, treated patients with 3 copies of SMN2 were between 3.3 and 15.1 months of age and had been in the study for an average of 8.74 months (range: 2 to 13.9 months). All patients were alive and free of permanent ventilation.
Ten of 15 patients were able to sit without support for at least 30 seconds, 4 patients were able to stand alone without support for at least 3 seconds, and 2 patients were able to walk at least five steps independently.
The follow up duration is too short to assess the development of patients treated with AVXS-101 treatment compared to the natural history of patients with 3 SMN2 copies, who have a heterogeneous clinical presentation. Therefore, no definitive conclusions about the benefit in this patient population can be drawn at this moment.
Onasemnogene abeparvovec has not been studied in patients with a bi-allelic mutation of the SMN1 gene and only one copy of SMN2 in clinical trials.
This medicinal product has been authorised under a so-called 'conditional approval' scheme. This means that further evidence on this medicinal product is awaited. The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.
The European Medicines Agency has deferred the obligation to submit the results of studies with onasemnogene abeparvovec in one or more subsets of the paediatric population in spinal muscular atrophy for the granted indication (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Onasemnogene abeparvovec vector shedding studies, which assess the amount of vector eliminated from the body through saliva, urine and faeces were performed.
Onasemnogene abeparvovec was detectable in shedding samples post-infusion. Clearance of onasemnogene abeparvovec was primarily via faeces and the majority is cleared within 30 days after dose administration. Onasemnogene abeparvovec concentrations in urine and saliva were 0.1% to 0.01% of initial concentration in the body at Day 1 post-infusion and dropped thereafter.
Biodistribution was evaluated in 2 patients who died 5.7 months and 1.7 months, respectively, after infusion of onasemnogene abeparvovec at the dose of 1.1 x 1014 vg/kg. Both cases showed that the highest levels of vector DNA were found in the liver. Vector DNA was also detected in the spleen, heart, pancreas, inguinal lymph node, skeletal muscles, peripheral nerves, kidney, lung, intestines, spinal cord, brain, and thymus. Immunostaining for SMN protein showed generalized SMN expression in spinal motor neurons, neuronal and glial cells of the brain, and in the heart, liver, skeletal muscles, and other tissues evaluated.
5.3. Preclinical safety data
Following intravenous administration in neonatal mice, vector and transgene were widely distributed with the highest expression generally observed in heart and liver, and substantial expression in the brain and spinal cord. In pivotal 3-month mouse toxicology studies, the main target organs of toxicity identified were the heart and liver. Onasemnogene abeparvovec-related findings in the ventricles of the heart were comprised of dose-related inflammation, oedema and fibrosis. In the atria of the heart, inflammation, thrombosis, myocardial degeneration/necrosis and fibroplasia were observed. Liver findings were comprised of hepatocellular hypertrophy, Kupffer cell activation, and scattered hepatocellular necrosis. A No Adverse Effect Level (NoAEL) was not identified for onasemnogene abeparvovec in mouse studies as ventricular myocardial inflammation/oedema/fibrosis and atrial inflammation were observed at the lowest dose tested (1.5 × 1014 vg/kg). This dose is regarded as the Maximum Tolerated Dose and approximately 1.4-fold the recommended clinical dose. Onasemnogene abeparvovec-related mortality was, in the majority of mice, associated with atrial thrombosis, and observed at 2.4 × 1014 vg/kg. The cause of the mortality in the rest of the animals was undetermined, although microscopic degeneration/regeneration in the hearts of these animals was found.
Genotoxicity, carcinogenicity and reproduction toxicity studies have not been conducted with onasemnogene abeparvovec.
In a toxicology study conducted in young adult non-human primates, administration of a single dose of 3 × 1013 vg/NHP (median dose 1.08 × 1013 vg/kg) onasemnogene abeparvovec intrathecally with Trendelenburg position, without corticosteroid treatment, resulted in minimal to marked mononuclear cell inflammation (primarily lymphocytes) in some dorsal root ganglia from all examined spinal cord levels, with neuronal satellitosis, neuronal necrosis, or complete neuronal loss with rare mineralization. The clinical relevance of this finding is unknown.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.