ZONOVATE Powder for solution, lyophilized Ref.[49705] Active ingredients: Octocog alfa
Source: Health Products and Food Branch (CA) Revision Year: 2021
Action and clinical pharmacology
10.1 Mechanism of Action
ZONOVATE temporarily replaces the missing clotting Factor VIII that is needed for effective hemostasis.
ZONOVATE contains human coagulation Factor VIII (rDNA), Antihemophilic Factor (Recombinant, B-Domain Truncated), a glycoprotein that has the same structure as human Factor VIII when activated, and post-translational modifications that are similar to those of the plasma-derived molecule.
When infused into a hemophilia patient, Factor VIII binds to endogenous von Willebrand Factor in the patient’s circulation. The two constituents of the Factor VIII/von Willebrand Factor complex (i.e. Factor VIII and von Willebrand Factor) have different physiological functions. Activated Factor VIII acts as a co-factor for activated Factor IX, accelerating the conversion of Factor X to activated Factor X. Activated Factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot can be formed. Hemophilia A is a sex-linked hereditary disorder of blood coagulation due to decreased levels of factor VIII:C and results in profuse bleeding into joints, muscles or internal organs, either spontaneously or as a result of accidental or surgical trauma. By replacement therapy the plasma levels of factor VIII are increased, thereby enabling a temporary correction of the factor deficiency and correction of bleeding tendencies.
10.2 Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia A. Determination of aPTT is a conventional in vitro assay for the biological activity of FVIII. Treatment with ZONOVATE normalizes the aPTT over the effective dosing period.
10.3 Pharmacokinetics
All pharmacokinetic studies with ZONOVATE were conducted in previously treated patients with severe hemophilia A (Factor VIII ≤1%). Plasma Factor VIII activity was measured using both the one-stage clotting assay and the chromogenic assay.
In a multi-center, multi-national, open-label, single dose pharmacokinetic study, 23 patients with severe hemophilia A received 50 international units/kg of ZONOVATE intravenously. Two patients were below the age of 18 years (13 and 17 years). The pharmacokinetic parameters for the 20 patients who completed the study are summarized in Table 1-8.
Table 1-8. Single-Dose Pharmacokinetics of ZONOVATE in Adult and Adolescent Patients with Severe Hemophilia A (Factor VIII ≤1%), Clotting Assay and Chromogenic Assay:
| Parameter | Clotting Assay (N=23) | Chromogenic Assay (N=20)* |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Incremental Recovery (IU/dL)/(IU/kg) | 1.9 (0.4) | 2.8 (0.6) |
| AUC (IU*h/dL) | 1364 (414) | 1870 (508) |
| CL (mL/h/kg) | 4.04 (1.43) | 2.87 (0.80) |
| t½ (h) | 10.69 (4.84) | 11.96 (9.28) |
| Vss (mL/kg) | 56.11 (13.28) | 44.31 (28.17) |
| Cmax (IU/dL) | 102 (21) | 154 (29) |
| MRT (h) | 15.22 (6.24) | 16.40 (10.14) |
AUC, area under the plasma concentration curve; CL, clearance; t1/2, terminal half-life; Vss, apparent volume of distribution at steady state; Cmax, maximum concentration; MRT, mean residence time.
* Samples for 3 of the 23 patients included in the study w ere not analyzed w ith the chromogenic assay.
In a separate pharmacokinetic study, 28 pediatric patients with severe hemophilia A (14 patients were below 6 years of age and 14 patients were between 6 to <12 years of age) received a single dose of 50 international units/kg of ZONOVATE. The pharmacokinetic parameters of ZONOVATE are summarized in Table 1-9 for both groups.
Table 1-9. Single-Dose Pharmacokinetics of ZONOVATE in 28 Pediatric Patients with Severe Hemophilia A (FVIII ≤1%), Clotting Assay and Chromogenic Assay:
| Parameter | Clotting assay | Chromogenic assay | ||
|---|---|---|---|---|
| 0 - <6 years | 6 - <12 years | 0 - <6 years | 6 - <12 years | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Incremental Recovery (IU/dL)/(IU/kg) | 1.8 (0.7) | 2.0 (0.4) | 2.2 (0.6) | 2.5 (0.6) |
| AUC (IU*h/dL) | 989 (414) | 1109 (373) | 1221 (438) | 1436 (348) |
| CL (mL/h/kg) | 6.26 (3.73) | 5.02 (1.67) | 4.60 (1.75) | 3.70 (1.00) |
| t½ (h) | 7.65 (1.84) | 8.02 (1.89) | 9.99 (1.71) | 9.42 (1.52) |
| Vss (mL/kg) | 57.30 (26.75) | 46.82 (10.62) | 55.79 (23.71) | 41.23 (6.00) |
| Cmax (IU/dL) | 100 (58) | 107 (35) | 112 (31) | 125 (27) |
| MRT (h) | 9.65 (2.46) | 9.91 (2.57) | 12.09 (1.88) | 11.61 (2.32) |
AUC, area under the plasma concentration curve; CL, clearance; t1/2, terminal half-life; Vss, apparent volume of distribution at steady state; Cmax, maximum concentration; MRT, mean residence time.
Some variation was observed in the pharmacokinetic parameters of ZONOVATE between pediatric and adult patients. The higher CL and the shorter t½ seen in pediatric patients compared to adult patients with hemophilia A may be due in part to the known higher plasma volume per kilogram body weight in younger patients. A single dose pharmacokinetic trial (50 IU/kg) was performed in 35 hemophilia patients (≥18 years of age) in different Body Mass Index (BMI) categories. The pharmacokinetic parameters of Zonovate are summarized in Table 1-10 and Table 1-11.
FVIII activity can be monitored with both the one stage clot and the chromogenic assay after ZONOVATE administration.
Table 1-10. Single-dose Pharmacokinetics of ZONOVATE (50 IU/kg) by BMI Classesa – Clotting assay – Mean (SD):
| Parameter | Underweight | Normal Weight | Overweight | Obese Class I | Obese Class II/III |
|---|---|---|---|---|---|
| N=5 | N=7 | N=8 | N=7 | N=7 | |
| Incremental Recovery (IU/dL)/(IU/kg) | 1.7 (0.2) | 2.0 (0.2) | 2.4 (0.4) | 2.3 (0.3)b | 2.6 (0.3) |
| AUC (IU*h/dL) | 1510 (360) | 1920 (610) | 1730 (610) | 2030 (840) | 2350 (590) |
| CL (mL/h/kg) | 3.91 (0.94) | 3.20 (1.00) | 3.63 (1.24) | 3.37 (1.79) | 2.51 (0.63) |
| t½ (h) | 11.3 (2.0) | 11.7 (3.5) | 9.4 (2.9) | 11.2 (3.5) | 11.1 (2.7) |
| Vss (mL/kg) | 56.8 (5.4) | 44.8 (6.5) | 39.6 (6.0) | 42.0 (9.0) | 35.0 (4.6) |
| Cmax (IU/dL) | 100 (11) | 121 (10) | 144 (26) | 140 (21) | 161 (32) |
| MRT (h) | 15.1 (3.0) | 15.3 (4.8) | 11.9 (3.7) | 14.4 (4.6) | 14.6 (3.7) |
a BMI groups: Underw eight: BMI <18.5 kg/m², Normal w eight: BMI 18.5-24.9 kg/m², Overw eight: BMI 25-29.9 kg/m², Obese class I: BMI 30-34.9 kg/m², Obese class II/III: BMI ≥35 kg/m².
b Based on 6 patients only.
MRT: mean residence time.
Table 1-11. Single-dose Pharmacokinetics of ZONOVATE (50 IU/kg) by BMI Classesa – Chromogenic assay – Mean (SD):
| Parameter | Underweight | Normal | Weight Overweight | Obese Class I | Obese Class II/III |
|---|---|---|---|---|---|
| N=5 | N=7 | N=9 | N=7 | N=7 | |
| Incremental Recovery (IU/dL)/(IU/kg) | 2.2 (0.4) | 2.9 (0.3) | 3.0 (0.5) | 3.2 (0.5) | 3.5 (0.5) |
| AUC (IU*h/dL) | 1860 (700) | 2730 (860) | 2310 (1020) | 2780 (1210) | 3050 (730) |
| CL (mL/h/kg) | 3.28 (0.87) | 2.25 (0.73) | 2.84 (1.09) | 2.58 (1.56) | 1.94 (0.52) |
| t½ (h) | 11.7 (2.4) | 11.5 (3.6) | 9.7 (3.4) | 10.4 (3.2) | 10.5 (2.5) |
| Vss (mL/kg) | 49.1 (10.4) | 31.2 (4.5) | 31.6 (5.8) | 28.9 (5.1) | 25.7 (4.0) |
| Cmax (IU/dL) | 138 (29) | 185 (24) | 194 (31) | 200 (33) | 227 (32) |
| MRT (h) | 15.5 (3.2) | 15.2 (4.9) | 12.6 (4.8) | 13.5 (4.6) | 13.9 (3.7) |
a BMI groups: Underw eight: BMI <18.5 kg/m², Normal w eight: BMI 18.5-24.9 kg/m², Overw eight: BMI 25-29.9 kg/m², Obese class I: BMI 30-34.9 kg/m², Obese class II/III: BMI ≥35 kg/m².
MRT: mean residence time.
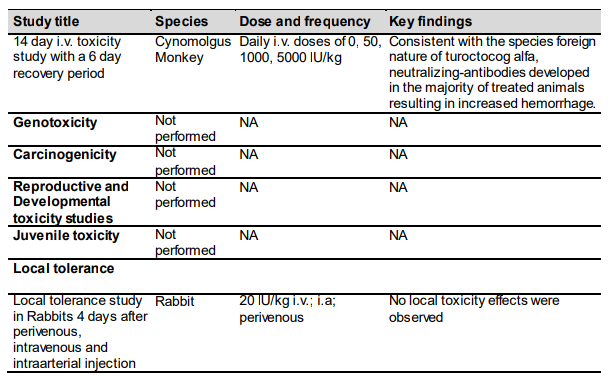
Toxicology
Non-clinical toxicology
Carcinogenicity, Genotoxicity, Reproductive Toxicity
Studies concerning carcinogenicity, genotoxicity and reproductive toxicity in animals have not been performed.
Table 2-6. Overview of non-clinical Toxicity Studies:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.