ZURZUVAE Capsule Ref.[107395] Active ingredients: Zuranolone
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
The mechanism of action of zuranolone in the treatment of PPD is not fully understood, but is thought to be related to its positive allosteric modulation of GABAA receptors.
12.2. Pharmacodynamics
Cardiac Electrophysiology
At two times the maximum recommended dose, ZURZUVAE does not cause clinically significant QTc interval prolongation.
Psychomotor Performance with Alcohol or Alprazolam
Co-administration of repeated 50 mg daily doses of ZURZUVAE with alcohol or alprazolam led to impairment in psychomotor performance [see Warnings and Precautions (5.2), Drug Interactions (7)].
12.3. Pharmacokinetics
Zuranolone exposure (Cmax and AUC) increased approximately dose proportionally with doses ranging from 30 mg to 60 mg (1.2 times of the recommended dosage of ZURZUVAE) with a moderate-fat meal (700 calories; 30% fat). Once-daily administration of ZURZUVAE resulted in accumulation of approximately 1.5-fold in systemic exposures and steady state was achieved in 3 to 5 days.
Absorption
Following oral administration, peak zuranolone concentrations occur at 5 to 6 hours (Tmax).
The absolute bioavailability of ZURZUVAE was not evaluated.
Effect of Food
Following administration of 30 mg of ZURZUVAE to healthy subjects, the Cmax increased by approximately 3.5-fold and the AUClast increased by approximately 1.8-fold with a low-fat meal (400 to 500 calories, 25% fat) compared to fasted conditions. The Cmax increased by approximately 4.3-fold and the AUClast increased by approximately 2-fold with a high-fat meal (800 to 1,000 calories, 50% fat) compared to fasted conditions. The Tmax was not impacted by food.
Distribution
The volume of distribution of zuranolone following oral administration is greater than 500 L. The mean blood-to-plasma concentration ratio ranged from 0.54 to 0.58. Plasma protein binding is greater than 99.5%.
Elimination
The terminal half-life of zuranolone is approximately 19.7 to 24.6 hours in an adult population. The mean apparent clearance (CL/F) of zuranolone is 33 L/h.
Metabolism
Zuranolone undergoes extensive metabolism, with CYP3A4 identified as a primary enzyme involved. There were no circulating human metabolites greater than 10% of total drug-related materials and none are considered to contribute to the therapeutic effects of zuranolone.
Excretion
Following oral administration of radiolabeled zuranolone, 45% of the dose was recovered in urine as metabolites with negligible unchanged zuranolone and 41% in feces as metabolites with less than 2% as unchanged zuranolone.
Specific Populations
Racial Groups
Black or African American participants had a 14% higher CL/F compared to participants of other races (Asian, White, or other).
Male and Female Patients, Patients with Renal Impairment, Patients with Hepatic Impairment
Exposures of zuranolone in specific populations are summarized in Figure 1 [see Dosage and Administration (2.4, 2.5) and Use in Specific Populations (8.6, 8.7)].
Figure 1. Effect of Specific Populations on the Pharmacokinetics of Zuranolone:
Data shown for participants ≥65 years relative to younger participants (18 to 45 years); female participants relative to male participants; renal and hepatic impairment relative to participants with normal renal and hepatic function, respectively. Hepatic impairment: Mild (Child-Pugh Class A); moderate (Child-Pugh Class B); severe (Child-Pugh Class C). Renal impairment: Mild (eGFR 60-89 mL/min/1.73m²); moderate (eGFR 30-59 mL/min/1.73m²); severe (eGFR <30 mL/min/1.73m² and not on dialysis).
CI = confidence interval; GMR = geometric mean ratio
Drug Interaction Studies
The effect of co-administered drugs on the pharmacokinetics of zuranolone is summarized in Figure 2 [see Dosage and Administration (2.3) and Drug Interactions (7)].
Figure 2. Effect of Co-Administered Drugs on the Pharmacokinetics of Zuranolone:
Data shown are zuranolone plus co-administered drug relative to zuranolone alone. CI = confidence interval; GMR = geometric mean ratio; *clinically significant drug interaction
ZURZUVAE did not affect the pharmacokinetics of alprazolam or ethanol. Increased impairment of psychomotor performance was observed when ZURZUVAE was co-administered with alprazolam or ethanol [see Warnings and Precautions (5.2) and Drug Interactions (7)].
In Vitro Studies
Enzyme systems: Zuranolone is not an inhibitor of CYP1A2, 2B6, 2C19, 2C8, 2C9, 2D6 or 3A4. Zuranolone is not an inducer for CYP1A2, CYP2B6 or CYP3A4 at the therapeutic dose range.
Transporter systems: Zuranolone is not a substrate of P-glycoprotein (P-gp), BCRP, OATP1B1 or OATP1B3. Zuranolone does not inhibit P-gp, BCRP, BSEP, OATP1B1, OATP1B3, OAT1, OAT2, OCT1, OCT2, MATE1, or MATE2.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
Oral administration of zuranolone (0, 3, 10, or 30 mg/kg/day) to male rats prior to and during mating with untreated females resulted in increased post-implantation loss and a corresponding decrease in the number of viable embryos at the high dose, which was also paternally toxic. There were no adverse effects on fertility or sperm parameters. Adverse effects on reproduction were not observed when males were remated after a 6-week treatment-free period. The no effect dose (10 mg/kg/day) for male reproductive toxicity was associated with a plasma zuranolone exposure (AUC) of approximately 4 times the human exposure at the MRHD. Oral administration of zuranolone (0, 1, 3, or 10 mg/kg/day) to female rats prior to and throughout mating and continuing through early gestation resulted in disruption of estrous cyclicity at the high dose, but there were no adverse effects on fertility or early embryonic development. The no-effect dose (3 mg/kg/day) for female reproductive toxicity was associated with exposures approximately 4 times that in humans at the MRHD.
Carcinogenesis
Oral administration of zuranolone in a 26-week carcinogenicity study in transgenic mice (0, 10, 30, or 100 mg/kg/day), and in a 104-week carcinogenicty study in rats (0, 2, 6, or 20 mg/kg/day in males and 0, 0.2, 0.6, or 1.5 mg/kg/day in females) was not associated with increases in tumors in either species. Plasma exposures (AUC) in rats at the highest dose tested were approximately 4 times that in humans at the maximum recommended human dose (MRHD) of 50 mg.
Mutagenesis
Zuranolone was not genotoxic when tested in an in vitro microbial mutagenicity (Ames) assay, an in vitro chromosome aberration assay in Chinese hamster ovary cells, and an in vivo bone marrow micronucleus assay in rats.
13.2. Animal Toxicology and/or Pharmacology
Death was observed in 1 dog four days after repeat dosing with 2.5 mg/kg for 9 months was stopped. Death/euthanasia in 2 dogs was also observed 2 to 4 days after dosing with 2.5 mg/kg for 14 days was stopped. Convulsions were observed in 1 dog three days after repeat dosing with 2.5 mg/kg for 14 days was stopped. Plasma exposure (AUC) at the no-effect dose in dogs was approximately 3 times the human exposure at the MRHD.
14. Clinical Studies
14.1 Postpartum Depression
The efficacy of ZURZUVAE for the treatment of postpartum depression (PPD) in adults was demonstrated in two randomized, placebo-controlled, double-blind, multicenter studies (Study 1, NCT04442503 and Study 2, NCT02978326) in women with PPD who met the Diagnostic and Statistical Manual of Mental Disorders criteria for a major depressive episode (DSM-5) with onset of symptoms in the third trimester or within 4 weeks of delivery. In these studies, concomitant use of existing oral antidepressants was allowed for patients taking a stable dose of oral antidepressant for at least 30 days before baseline. These studies included patients with HAMD-17 scores ≥26 at baseline.
In Study 1, patients received 50 mg of ZURZUVAE (N=98) or placebo (N=97) once daily in the evening with fat-containing food for 14 days, with the option to reduce the dosage based on tolerability to 40 mg once daily of ZURZUVAE or placebo. The patients were followed for a minimum of 4 weeks after the 14-day treatment course.
In Study 2, patients received another zuranolone capsule formulation (approximately equivalent to 40 mg of ZURZUVAE) (N=76) or placebo (N=74) once daily in the evening with food for 14 days. The patients were followed for a minimum of 4 weeks after the 14-day treatment course.
Baseline Demographics and Disease Characteristics
In Studies 1 and 2, the baseline demographic and disease characteristics of patients were similar between the ZURZUVAE and placebo groups. In Study 1, patients had a mean age of 30 years (range 19 to 44 years); were 70% White, 22% Black or African American, 1% Asian, and 7% were other races; and 38% were of Hispanic or Latino ethnicity. Baseline use of stable oral antidepressants was reported in 15% of patients. In Study 2, patients had a mean age of 28 years (range 18 to 44 years); were 56% White, 41% Black or African American, 1% Asian, and 2% were other races; and 23% were of Hispanic or Latino ethnicity. Baseline use of stable oral antidepressants was reported in 19% of patients.
The primary endpoint for Studies 1 and 2 was the change from baseline in depressive symptoms as measured by the HAMD-17 total score at Day 15. In these studies, patients in the ZURZUVAE groups experienced statistically significantly greater improvement on the primary endpoint compared to patients in the placebo groups, as shown in Table 5. For Study 1, the key secondary endpoints included change from baseline in HAMD-17 total score at Days 3, 28, and 45.
Table 5. Results for the Primary Endpoint: Change from Baseline in the HAMD-17 Total Score at Day 15 (Studies 1 and 2 in Women with PPD):
| Study Number | Treatment Group | N | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Difference (95% CI) |
|---|---|---|---|---|---|
| 1 | 50 mg of ZURZUVAE | 98 | 28.6 (2.49) | -15.6 (0.82) | -4.0 (-6.3, -1.7) |
| Placebo | 97 | 28.8 (2.34) | -11.6 (0.82) | ||
| 2 | Zuranolone (another capsule formulation)* | 76 | 28.4 (2.09) | -17.8 (1.04) | -4.2 (-6.9, -1.5) |
| Placebo | 74 | 28.8 (2.32) | -13.6 (1.07) |
HAMD-17: 17-item Hamilton depression rating scale; SD: standard deviation; LS: least squares; SE: standard error; CI: confidence interval
* This capsule formulation of zuranolone is approximately equivalent to 40 mg of ZURZUVAE.
Subgroup analyses of the primary endpoint did not suggest differences in response to 50 mg of ZURZUVAE for age, race, or BMI.
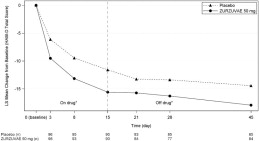
The time course of response for 50 mg ZURZUVAE compared to placebo for Study 1 is shown in Figure 3.
Figure 3. Mean Change from Baseline in HAM-D Total Score Over Time (Days) in Women with PPD in Study 1:
* Both phases were double-blind.
14.2 Effects on Driving
Two randomized, double-blind, placebo- and active-controlled, four-way crossover studies (Study 3 and Study 4) evaluated the effects of nighttime ZURZUVAE administration on next-morning driving performance, 9 hours after dosing, using a computer-based driving simulation.
Study 3
In Study 3, 50 mg of ZURZUVAE was administered for six consecutive nights and on the seventh night a single dose of 50 mg or 100 mg (two times the recommended dose) was administered. The primary driving performance outcome measure was the change in Standard Deviation of Lateral Position (SDLP) (a measure of driving impairment) in the ZURZUVAE group compared to the placebo group on Days 2 and 8 (after a single dose and repeat doses, respectively).
Study 3 included 67 healthy participants. The median age was 45 years old (age ranged from 22 to 81 years old; 7 participants were ≥ 65 years of age); there were 38 males and 29 females; 88% were White, 5% were Black or African American, 3% were Asian, and 5% were other races; and 12% were of Hispanic/Latino ethnicity.
A single 50 mg dose of ZURZUVAE caused statistically significant impairment in next-morning driving performance compared to placebo. Statistically significant effects on driving were also observed on Day 8 following daily administration of 50 mg of ZURZUVAE. Administration of 100 mg of ZURZUVAE (twice the maximum recommended dose) on the final night increased impairment in driving ability [see Warnings and Precautions (5.1)].
The exposure-response analysis for driving impairment in Study 3 suggested that the projected mean placebo-adjusted SDLP at 12 hours post-dose would be less than the threshold associated with driving impairment.
Study 4
In Study 4, 30 mg of ZURZUVAE (0.6 times the maximum recommended daily dose) was administered for four consecutive nights and on the fifth night a single dose of 30 mg or 60 mg (1.2 times the recommended daily dose) was administered. The primary driving performance outcome measure was the change in SDLP in the ZURZUVAE group compared to the placebo group on Days 2 and 6 (after a single dose and repeat doses, respectively).
Study 4 included 60 participants; 60% and 40% were male and female, respectively; the median age was 41 years old (range was 22 to 62 years old); 90% were White, 5% were Black or African American, 3% were Asian, and 2% were other races; and 15% were of Hispanic/Latino ethnicity.
A single 30 mg dose of ZURZUVAE caused a statistically significant impairment in next-morning driving performance compared to placebo. The mean effect on driving performance was not statistically significantly different following 30 mg of ZURZUVAE compared to placebo on Day 6; however, driving ability was impaired in some participants taking ZURZUVAE. Administration of 60 mg of ZURZUVAE (1.2 times the maximum recommended dose) on the final night caused statistically significant impairment in next-morning driving performance compared to placebo [see Warnings and Precautions (5.1)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.