ZYCLARA Cream Ref.[11107] Active ingredients: Imiquimod
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
The mechanism of action of ZYCLARA Cream in treating AK and EGW lesions is unknown.
12.2. Pharmacodynamics
The pharmacodynamics of ZYCLARA Cream are unknown.
Imiquimod is a Toll-like receptor 7 agonist that activates immune cells. Topical application to skin is associated with increases in markers for cytokines and immune cells.
Actinic Keratosis
In a study of 18 subjects with AK comparing imiquimod cream, 5% to vehicle, increases from baseline in Week 2 biomarker levels were reported for CD3, CD4, CD8, CD11c, and CD68 for imiquimod cream, 5% treated subjects; however, the clinical relevance of these findings is unknown.
External Genital Warts
Imiquimod has no direct antiviral activity in cell culture.
12.3. Pharmacokinetics
Following dosing with two packets of ZYCLARA Cream, 3.75% once daily (18.75 mg imiquimod/day) for up to 3 weeks, systemic absorption of imiquimod was observed in all subjects when ZYCLARA Cream was applied to the face and/or scalp in 17 subjects with at least 10 AK lesions. The mean peak serum imiquimod concentration at the end of the trial was approximately 0.323 ng/mL. The median time to maximal concentrations (Tmax) occurred at 9 hours after dosing. Based on the plasma half-life of imiquimod observed at the end of the study, 29.3±17.0 hours, steady-state concentrations can be anticipated to occur by Day 7 with once-daily dosing.
Systemic absorption of imiquimod (up to 9.4 mg [one packet]) across the affected skin of 18 subjects with EGW was observed with once daily dosing for 3 weeks in all subjects. The subjects had either a minimum of 8 warts (range 8-93) or a surface area involvement of greater than 100 mm² (range 15-620 mm²) at study entry. The mean peak serum imiquimod concentration at Day 21 was 0.488 +/- 0.368 ng/mL. The median time to maximal concentrations (Tmax) occurred 12 hours after dosing. Based on the plasma half-life of imiquimod observed at the end of the study, 24.1 +/- 12.4 hours, steady-state concentrations can be anticipated to occur by day 7 with once daily dosing. Because of the small number of subjects present (13 males, 5 females) it was not possible to select out or do an analysis of absorption based on gender/site of application.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) rat carcinogenicity study, imiquimod was administered to Wistar rats on a 2X/week (up to 6 mg/kg/day) or daily (3 mg/kg/day) dosing schedule for 24 months. No treatment-related tumors were noted in the oral rat carcinogenicity study up to the highest doses tested in this study of 6 mg/kg administered 2X/week in female rats (7.1X MRHD based on weekly AUC comparisons), 4 mg/kg administered 2X/week in male rats (6.1X MRHD based on weekly AUC comparisons) or 3 mg/kg administered 7X/week to male and female rats (12X MRHD based on weekly AUC comparisons).
In a dermal mouse carcinogenicity study, imiquimod cream (up to 5 mg/kg/application imiquimod or 0.3% imiquimod cream) was applied to the backs of mice 3X/week for 24 months. A statistically significant increase in the incidence of liver adenomas and carcinomas was noted in high-dose male mice compared to control male mice (21X MRHD based on weekly AUC comparisons). An increased number of skin papillomas was observed in vehicle cream control group animals at the treated site only.
In a 52-week dermal photocarcinogenicity study, the median time to onset of skin tumor formation was decreased in hairless mice following chronic topical dosing (3X/week; 40 weeks of treatment followed by 12 weeks of observation) with concurrent exposure to UV radiation (5 days per week) with vehicle alone. No additional effect on tumor development beyond the vehicle effect was noted with the addition of the active ingredient, imiquimod, to the vehicle cream.
Imiquimod revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, mouse lymphoma L5178Y assay, Chinese hamster ovary cell chromosome aberration assay, human lymphocyte chromosome aberration assay and SHE cell transformation assay) and three in vivo genotoxicity tests (rat and hamster bone marrow cytogenetics assay and a mouse dominant lethal test).
Daily oral administration of imiquimod to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 25X MRHD based on AUC comparisons.
14. Clinical Studies
14.1 Actinic Keratosis
In two double-blind, randomized, vehicle-controlled clinical studies, 479 subjects with AK were treated with ZYCLARA Cream, 3.75%, ZYCLARA Cream, 2.5%, or vehicle cream. Studies enrolled subjects 18 years of age or older with 5 to 20 typical visible or palpable AK lesions of the face or scalp. Study cream was applied to either the entire face (excluding ears) or balding scalp once daily for two 2-week treatment cycles separated by a 2-week no-treatment period. Subjects then continued in the study for an 8-week follow-up period during which they returned for clinical observations and safety monitoring. Study subjects ranged from 36 to 90 years of age and 54% had Fitzpatrick skin type I or II. All ZYCLARA Cream-treated subjects were Caucasians.
On a scheduled dosing day, up to two packets of the study cream were applied to the entire treatment area prior to normal sleeping hours and left on for approximately 8 hours. Efficacy was assessed by AK lesion counts at the 8-week post-treatment visit. All AKs in the treatment area were counted, including baseline lesions as well as lesions which appeared during therapy.
Complete clearance required absence of any lesions including those that appeared during therapy in the treatment area. Complete and partial clearance rates are shown in the tables below. Partial clearance rate was defined as the percentage of subjects in whom the number of baseline AKs was reduced by 75% or more. The partial clearance rate was measured relative to the numbers of AK lesions at baseline.
Table 5. Rate of Subjects with Complete Clearance at 8 Weeks Post-Treatment:
| ZYCLARA Cream, 3.75% | ZYCLARA Cream, 2.5% | Vehicle Cream | |
|---|---|---|---|
| Study AK1 | 26% (21/81) | 23% (19/81) | 3% (2/80) |
| Study AK2 | 46% (36/79) | 38% (30/79) | 10% (8/79) |
Table 6. Rate of Subjects with Partial Clearance (≥75%) at 8 Weeks Post-Treatment:
| ZYCLARA Cream, 3.75% | ZYCLARA Cream, 2.5% | Vehicle Cream | |
|---|---|---|---|
| Study AK1 | 46% (37/81) | 42% (34/81) | 19% (15/80) |
| Study AK2 | 73% (58/79) | 54% (43/79) | 27% (21/79) |
During the course of treatment, 86% (138/160) of ZYCLARA Cream, 3.75% subjects and 84% (135/160) of ZYCLARA Cream, 2.5% subjects experienced a transient increase in lesions evaluated as actinic keratoses relative to the number present at baseline within the treatment area.
14.2 External Genital Warts
In two double-blind, randomized, placebo-controlled clinical studies, 601 subjects with EGW were treated with 3.75% imiquimod cream, or a matching placebo cream. Studies enrolled subjects aged from 15 to 81 years. The baseline wart area ranged from 6 to 5579 mm² (median 60 mm²) and the baseline wart count ranged from 2 to 48 warts. Most subjects had two or more treated anatomic areas at baseline. Anatomic areas included: inguinal, perineal, and perianal areas (both genders); the glans penis, penis shaft, scrotum, and foreskin (in men); and the vulva (in women). Up to one packet of study cream was applied once daily. The study cream was applied to all warts prior to normal sleeping hours and left on for approximately 8 hours. Subjects continued applying the study cream for up to 8 weeks, stopping if they achieved complete clearance of all (baseline and new) warts in all anatomic areas. Subjects who achieved complete clearance of all warts at any time up to the Week 16 visit entered a 12-week follow-up period to assess recurrence.
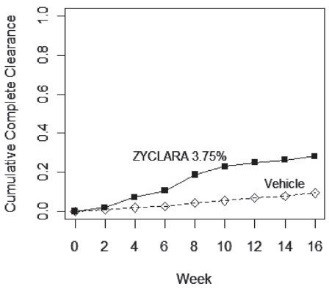
Complete clearance was defined as clearance of all warts (baseline and new) in all anatomic areas within 16 weeks from baseline. The complete clearance rates are shown in Table 7. The proportions of subjects who achieved complete clearance at or before a given week (cumulative proportion) for the combined studies are shown in Figure 1. Complete clearance rates by gender for the combined studies are shown in Table 8.
Table 7. Percent of Subjects with Complete Clearance of External Genital Warts Within 16 Weeks from Baseline:
| ZYCLARA Cream, 3.75% | Vehicle Cream | |
|---|---|---|
| Study EGW1 | 53/195 (27%) | 10/97 (10%) |
| Study EGW2 | 60/204 (29%) | 9/105 (9%) |
Figure 1. Cumulative Proportion of Subjects Achieving Complete Clearance of External Genital Warts by a Given Week (Combined Studies):
Table 8. Percent of Subjects with Complete Clearance of External Genital Warts within 16 Weeks from Baseline by Gender (Combined Studies):
| ZYCLARA Cream, 3.75% | Vehicle Cream | |
|---|---|---|
| Females | 79/216 (37%) | 15/106 (14%) |
| Males | 34/183 (19%) | 4/96 (4%) |
Of the 113 ZYCLARA Cream, 3.75%-treated subjects who achieved complete clearance in the two studies, 17 (15%) subjects had a recurrence within 12 weeks.
No studies were conducted directly comparing the 3.75% and 5% concentrations of imiquimod cream in the treatment of external genital warts.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.