ZYKADIA Hard capsule Ref.[6632] Active ingredients: Ceritinib
Source: European Medicines Agency (EU) Revision Year: 2018 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: antineoplasic and immunomodulating agents
ATC code: L01XE28
Mechanism of action
Ceritinib is an orally highly selective and potent ALK inhibitor. Ceritinib inhibits autophosphorylation of ALK, ALK-mediated phosphorylation of downstream signalling proteins and proliferation of ALK-dependent cancer cells both in vitro and in vivo.
ALK translocation determines expression of the resulting fusion protein and consequent aberrant ALK signaling in NSCLC. In the majority of NSCLC cases, EML4 is the translocation partner for ALK; this generates an EML4-ALK fusion protein containing the protein kinase domain of ALK fused to the N-terminal part of EML4. Ceritinib was demonstrated to be effective against EML4-ALK activity in a NSCLC cell line (H2228), resulting in inhibition of cell proliferation in vitro and regression of tumours in H2228-derived xenografts in mouse and rat.
Clinical efficacy and safety
Previously untreated ALK-positive advanced NSCLC - randomised phase 3 Study A2301 (ASCEND-4)
The efficacy and safety of Zykadia for the treatment of advanced ALK-positive NSCLC patients who have not received previous systemic treatment anti-cancer therapy (including ALK inhibitor) with the exception of neo-adjuvant or adjuvant therapy, was demonstrated in a global multicentre, randomised, open-label phase 3 Study A2301.
A total of 376 patients were randomised in a 1:1 ratio (stratified by WHO performance status, prior adjuvant/neoadjuvant chemotherapy and presence/absence of brain metastasis at screening) to receive either ceritinib (750 mg daily, fasted) or chemotherapy (based on investigator's choice - pemetrexed [500 mg/m²] plus cisplatin [75 mg/m²] or carboplatin [AUC 5-6], administered every 21 days). Patients who completed 4 cycles of chemotherapy (induction) without progressive disease subsequently received pemetrexed (500 mg/m²) as single-agent maintenance therapy every 21 days. One hundred and eighty-nine (189) patients were randomised to ceritinib and one hundred eightyseven (187) were randomised to chemotherapy.
The median age was 54 years (range: 22 to 81 years); 78.5% of patients were younger than 65 years. A total of 57.4% of patients were female. 53.7% of the study population was Caucasian, 42.0% Asian, 1.6% Black and 2.6% other races The majority of patients had adenocarcinoma (96.5%) and had either never smoked or were former smokers (92.0%). The Eastern Cooperative Oncology Group (ECOG) performance status was 0/1/2 in 37.0%/56.4%/6.4% of patients, and 32.2% had brain metastasis at baseline. 59.5% of patients with brain metastasis at baseline received no prior radiotherapy to the brain. Patients with symptomatic CNS (central nervous system) metastases who were neurologically unstable or had required increasing doses of steroids within the 2 weeks prior to screening to manage CNS symptoms, were excluded from the study.
Patients were allowed to continue the assigned study treatment beyond initial progression in case of continued clinical benefit as per the investigator's opinion. Patients randomised to the chemotherapy arm could cross-over to receive ceritinib upon RECIST-defined disease progression confirmed by blinded independent review committee (BIRC). One hundred and five (105) patients out of the 145 patients (72.4%) that discontinued treatment in the chemotherapy arm received subsequent ALK inhibitor as first antineoplastic therapy. Of these patients 81 received ceritinib.
The median duration of follow-up was 19.7 months (from randomisation to cut-off date).
The study met its primary objective demonstrating a statistically significant improvement in progression free survival (PFS) by BIRC (see Table 3 and Figure 1). The PFS benefit of ceritinib was consistent by investigator assessment and across various subgroups including age, gender, race, smoking class, ECOG performance status and disease burden.
The overall survival (OS) data was not mature with 107 deaths representing approximately 42.3% of the required events for the final OS analysis.
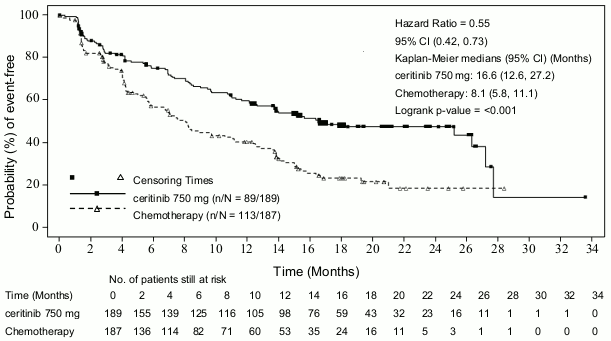
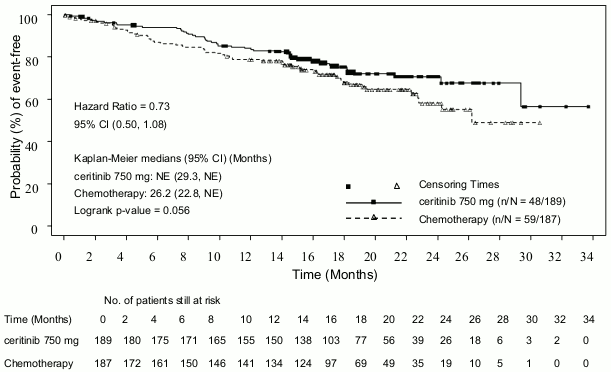
Efficacy data from Study A2301 are summarised in Table 3, and the Kaplan-Meier curves for PFS and OS are shown in Figure 1 and Figure 2, respectively.
Table 3. ASCEND-4 (Study A2301) - Efficacy results in patients with previously untreated ALK-positive advanced NSCLC:
| Ceritinib (N=189) | Chemotherapy (N=187) | |
|---|---|---|
| Progression-free survival (based on BIRC) | ||
| Number of events, n (%) | 89 (47.1) | 113 (60.4) |
| Median, monthsd (95% CI) | 16.6 (12.6, 27.2) | 8.1 (5.8, 11.1) |
| HR (95% CI)a | 0.55 (0.42, 0.73) | |
| p-valueb | <0.001 | |
| Overall survivalc | ||
| Number of events, n (%) | 48 (25.4) | 59 (31.6) |
| Median, monthsd (95% CI) | NE (29.3, NE) | 26.2 (22.8, NE) |
| OS rate at 24 monthsd, % (95% CI) | 70.6 (62.2, 77.5) | 58.2 (47.6, 67.5) |
| HR (95% CI)a | 0.73 (0.50,1.08) | |
| p-valueb | 0.056 | |
| Tumour response (based on BIRC) | ||
| Overall response rate (95% CI) | 72.5% (65.5, 78.7) | 26.7% (20.5, 33.7) |
| Duration of response (based on BIRC) | ||
| Number of responders | 137 | 50 |
| Median, monthsd (95% CI) | 23.9 (16.6, NE) | 11.1 (7.8, 16.4) |
| Event-free rate at 18 monthsd, % (95% CI) | 59.0 (49.3, 67.4) | 30.4 (14.1, 48.6) |
HR=hazard ratio; CI=confidence interval; BIRC=Blinded Independent Review Committee; NE=not estimable
a Based on the Cox proportional hazards stratified analysis.
b Based on the stratified log-rank test.
c OS analysis was not adjusted for the effects of cross-over.
d Estimated using the Kaplan-Meier method.
Figure 1. ASCEND-4 (Study A2301) - Kaplan-Meier curves of progression-free survival as assessed by BIRC:
Figure 2. ASCEND-4 (Study A2301)- Kaplan-Meier plot of overall survival by treatment arm:
Patient reported outcome questionnaires (Lung cancer symptom scale [LCSS], EORTC-QLQ-C30 [C30], EORTC QLQ-LC13 [LC13] and EQ-5D-5L) were completed by 80% or more of patients in the ceritinib and chemotherapy arms for all questionnaires at most of the time-points during the course of the study.
Ceritinib significantly prolonged time to deterioration for the pre-specified lung cancer specific symptoms of interest of cough, pain and dyspnoea (composite endpoint LCSS: HR=0.61, 95% CI: 0.41, 0.90, median Time to Deterioration [TTD] NE [95% CI: 20.9, NE] in the ceritinib arm versus 18.4 months [13.9, NE] in the chemotherapy arm; LC13: HR=0.48, 95% CI: 0.34, 0.69, median TTD 23.6 months [95% CI: 20.7, NE] in the ceritinib arm versus 12.6 months [95% CI: 8.9, 14.9] in the chemotherapy arm).
Patients receiving ceritinib showed significant improvements over chemotherapy in general Quality of Life and global Health Status measures (LCSS [p<0.001], QLQ-C30, [p<0.001] and EQ-5D-5L index [p<0.001]).
In Study A2301, 44 patients with measurable brain metastasis at baseline and at least one post-baseline brain radiological assessment (22 patients in the ceritinib arm and 22 patients in the chemotherapy arm) were assessed for intracranial response by BIRC neuro-radiologist per modified RECIST 1.1 (i.e. up to 5 lesions in the brain). The overall intracranial response rate (OIRR) was higher with ceritinib (72.7%, 95% CI: 49.8, 89.3) as compared to the chemotherapy arm (27.3%, 95% CI: 10.7, 50.2). The median PFS by BIRC using RECIST 1.1 was longer in the ceritinib arm compared to the chemotherapy arm in both subgroups of patients with brain metastases and without brain metastases.
The median PFS in patients with brain metastases was 10.7 months (95% CI: 8.1, 16.4) versus 6.7 months (95% CI: 4.1, 10.6) in the ceritinib and chemotherapy arms, respectively, with HR=0.70 (95% CI: 0.44, 1.12). The median PFS in patients without brain metastases was 26.3 months (95% CI: 15.4, 27.7) versus 8.3 months (95% CI: 6.0, 13.7) in the ceritinib and chemotherapy arms, respectively, with HR=0.48 (95% CI: 0.33, 0.69).
Previously treated ALK-positive advanced NSCLC - randomised phase 3 Study A2303 (ASCEND-5)
The efficacy and safety of Zykadia for the treatment of ALK-positive advanced NSCLC patients who have received previous treatment with crizotinib, was demonstrated in a global multicentre, randomised, open-label phase 3 Study A2303.
A total of 231 patients with advanced ALK positive NSCLC who have received prior treatment with crizotinib and chemotherapy (one or two regimen including a platinum-based doublet) were included in the analysis. One hundred fifteen (115) patients were randomised to Zykadia and one hundred sixteen (116) were randomised to chemotherapy (either pemetrexed or docetaxel). Seventy-three (73) patients received docetaxel and 40 received pemetrexed. In the ceritinib arm, 115 patients were treated with 750 mg once daily fasted. The median age was 54.0 years (range: 28 to 84 years); 77.1% of patients were younger than 65 years. A total of 55.8% of patients were female. 64.5% of the study population were Caucasian, 29.4% Asian, 0.4% Black and 2.6% other races. The majority of patients had adenocarcinoma (97.0%) and had either never smoked or were former smokers (96.1%). The ECOG performance status was 0/1/2 in 46.3%/47.6%/6.1% of patients respectively, and 58.0% had brain metastasis at baseline. All patients were treated with prior crizotinib. All except one patient received prior chemotherapy (including a platinum doublet) for advanced disease; 11.3% of the patients in the ceritinib arm and 12.1% of the patients in the chemotherapy arm were treated with two prior chemotherapy regimen for advanced disease.
Patients were allowed to continue the assigned study treatment beyond initial progression in case of continued clinical benefit as per the investigator's opinion. Patients randomised to the chemotherapy arm could further crossover to receive Zykadia upon RECIST-defined disease progression confirmed by BIRC.
The median duration of follow-up was 16.5 months (from randomisation to data cut-off date).
The study met its primary objective demonstrating a statistically significant improvement in PFS by BIRC with an estimated 51% risk reduction in the ceritinib arm compared to chemotherapy arm (see Table 4 and Figure 3). The PFS benefit of Zykadia was consistent across various subgroups including age, gender, race, smoking class, ECOG performance status, and presence of brain metastases or prior response to crizotinib. The PFS benefit was further supported by local investigator assessment, and analysis of overall response rate (ORR) and disease control rate (DCR).
OS data was immature with 48 (41.7%) events in the ceritinib arm and 50 (43.1%) events in the chemotherapy arm, corresponding to approximately 50% of the required events for the final OS analysis. In addition, 81 patients (69.8%) in the chemotherapy arm received subsequent Zykadia as first antineoplastic therapy after study treatment discontinuation.
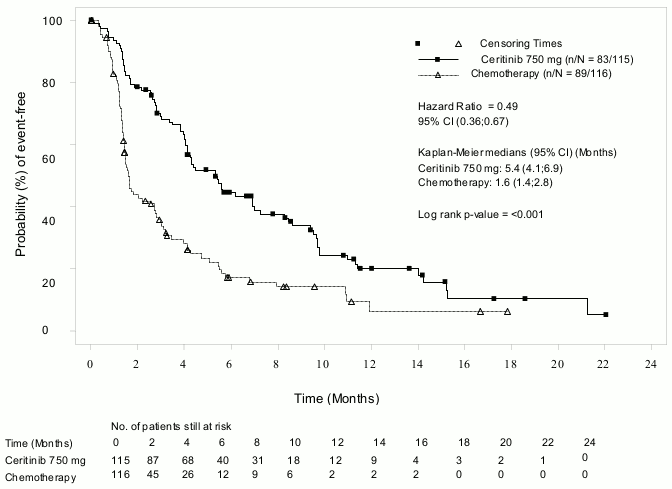
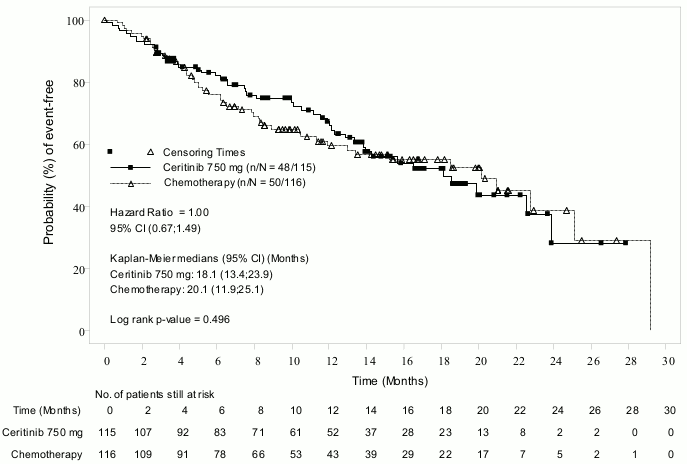
Efficacy data from Study A2303 are summarised in Table 4, and the Kaplan-Meier curves for PFS and OS are shown in Figure 3 and 4, respectively.
Table 4. ASCEND-5 (Study A2303) – Efficacy results in patients with previously treated ALK-positive metastatic/advanced NSCLC:
| Ceritinib (N=115) | Chemotherapy (N=116) | |
|---|---|---|
| Duration of follow-up | 16.5 | |
| Median (months) (min–max) | (2.8–30.9) | |
| Progression-free survival (based on BIRC) | ||
| Number of events, n (%) | 83 (72.2%) | 89 (76.7%) |
| Median, months (95% CI) | 5.4 (4.1, 6.9) | 1.6 (1.4, 2.8) |
| HR (95% CI)a | 0.49 (0.36, 0.67) | |
| p-valueb | <0.001 | |
| Overall survivalc | ||
| Number of events, n (%) | 48 (41.7%) | 50 (43.1%) |
| Median, months (95% CI) | 18.1 (13.4, 23.9) | 20.1 (11.9, 25.1) |
| HR (95% CI)a | 1.00 (0.67,1.49) | |
| p-valueb | 0.496 | |
| Tumour responses (based on BIRC) | ||
| Objective response rate (95% CI) | 39.1% (30.2, 48.7) | 6.9% (3.0, 13.1) |
| Duration of response | ||
| Number of responders | 45 | 8 |
| Median, monthsd (95% CI) | 6.9 (5.4, 8.9) | 8.3 (3.5, NE) |
| Event-free probability estimate at 9 monthsd (95% CI) | 31.5% (16.7%, 47.3%) | 45.7% (6.9%, 79.5%) |
HR=hazard ratio; CI=confidence interval; BIRC=Blinded Independent Review Committee; NE=not estimable
a Based on the stratified Cox proportional hazards analysis.
b Based on the stratified log-rank test.
c OS analysis was not adjusted for the potentially confounding effects of cross over.
d Estimated using the Kaplan-Meier method.
Figure 3. ASCEND-5 (Study A2303) – Kaplan-Meier plot of progression-free survival as assessed by BIRC:
Figure 4. ASCEND-5 (Study A2303) – Kaplan-Meier plot of overall survival by treatment arm:
Patient reported outcome questionnaires were collected using the EORTC QLQ C30/LC13, LCSS and EQ-5D-5L. 75% or more of patients in the ceritinib and chemotherapy arms completed the LCSS questionnaires at most of the time points during the course of the study. Significant improvements were reported for the majority of lung cancer specific symptoms for Zykadia compared to chemotherapy (four out of six LCSS and 10 out of 12 QLQ-LC13 symptom scores). Ceritinib significantly prolonged time to deterioration for the lung cancer specific symptoms of interest of cough, pain and dyspnoea (composite endpoint LCSS: HR=0.40; 95% CI: 0.25, 0.65, median Time to Deterioration [TTD] 18.0 months [95% CI: 13.4, NE] in the ceritinib arm versus 4.4 months [95% CI: 1.6, 8.6] in the chemotherapy arm; LC13: HR=0.34; 95% CI: 0.22, 0. 52, median TTD 11.1 months [95% CI: 7.1, 14.2] in the ceritinib arm versus 2.1 months [95% CI: 1.0, 5.6] in the chemotherapy arm). The EQ-5D questionnaire showed a significant overall health status improvement for Zykadia in comparison to the chemotherapy.
In Study A2303, 133 patients with baseline brain metastasis (66 patients in the Zykadia arm and 67 patients in the chemotherapy arm) were assessed for intracranial response by BIRC neuroradiologist (per modified RECIST 1.1 (i.e. up to 5 lesions in the brain). The OIRR in patients with measurable disease in the brain at baseline and at least one post-baseline assessment was higher in the ceritinib arm (35.3%, 95% CI: 14.2, 61.7) compared to the chemotherapy arm (5.0%, 95% CI: 0.1, 24.9). The median PFS by BIRC using RECIST 1.1 was longer in the ceritinib arm compared to the chemotherapy arm in both subgroups of patients with brain metastases and without brain metastases. The median PFS in patients with brain metastases was 4.4 months (95% CI: 3.4, 6.2) versus 1.5 months (95% CI: 1.3, 1.8) in the ceritinib and chemotherapy arms, respectively with HR=0.54 (95% CI: 0.36, 0.80). The median PFS in patients without brain metastases was 8.3 months (95% CI: 4.1, 14.0) versus 2.8 months (95% CI: 1.4, 4.1) in the ceritinib and chemotherapy arms, respectively with HR=0.41 (95% CI: 0.24, 0.69).
Dose optimisation Study A2112 (ASCEND-8)
The efficacy of Zykadia 450 mg with food was evaluated in a multicentre, open-label dose optimisation study A2112 (ASCEND-8). A total of 81 previously untreated patients with ALK-positive locally advanced or metastatic NSCLC were randomised to receive Zykadia 450 mg once daily with food (N=41) or Zykadia 750 mg once daily under fasted conditions (N=40). A key secondary efficacy endpoint was ORR according to RECIST 1.1 as evaluated by BIRC.
The population characteristics across the two arms were: mean age 53 years, age less than 65 (79%), female (57%), Caucasian (54%), Asian (33%), never or former smoker (95%), WHO PS 0 or 1 (93%), adenocarcinoma histology (94%), and metastases to the brain (33%).
Efficacy results from ASCEND-8 are summarised in Table 5 below.
Table 5. ASCEND-8 (Study A2112) - Efficacy results in patients with previously untreated ALK-positive locally advanced or metastatic NSCLC by BIRC:
| Efficacy Parameter | Ceritinib 450 mg with food (N=41) | Ceritinib 750 mg fasted (N=40) |
|---|---|---|
| Overall Response Rate (ORR: CR+PR), n (%) | 32 (78.0) | 28 (70.0) |
| (95% CI)a | (62.4, 89.4) | (53.5, 83.4) |
CI: Confidence Interval
Complete Response (CR), Partial Response (PR) confirmed by repeat assessments performed not less than 4 weeks after response criteria were first met
Overall response rate determined based on BIRC assessment per RECIST 1.1
a Exact binomial 95% confidence interval
Single arm studies X2101 and A2201
The use of Zykadia in the treatment of ALK-positive NSCLC patients previously treated with an ALK inhibitor was investigated in two global, multicentre, open-label, single-arm phase ½ studies (Study X2101 and Study A2201).
In study X2101 a total of 246 ALK-positive NSCLC patients were treated at a Zykadia dose of 750 mg once daily fasted: 163 who had received prior treatment with an ALK inhibitor and 83 who were ALK inhibitor naïve. Of the 163 ALK-positive NSCLC patients who had received prior treatment with an ALK inhibitor, the median age was 52 years (range: 24-80 years); 86.5% were younger than 65 years and 54% were female. The majority of patients were Caucasian (66.3%) or Asian (28.8%). 93.3% had adenocarcinoma and 96.9% had either never been or were former smokers. All of the patients were treated with at least one regimen prior to enrolment into the study and 84.0% with two or more regimens.
Study A2201 involved 140 patients who had been previously treated with 1-3 lines of cytotoxic chemotherapy followed by treatment with crizotinib, and who had then progressed on crizotinib. The median age was 51 years (range: 29-80 years); 87.1% of patients were younger than 65 years and 50.0% were female. The majority of patients were Caucasian (60.0%) or Asian (37.9%). 92.1% of patients had adenocarcinoma.
The main efficacy data for both studies are summarised in Table 6. Final overall survival (OS) data are presented for Study A2201. For Study X2101, OS data were not yet mature at the time of the analysis.
Table 6 ALK-positive advanced NSCLC - overview of efficacy results from Studies X2101 and A2201:
| Study X2101 ceritinib 750 mg | Study A2201 ceritinib 750 mg | |
|---|---|---|
| N=163 | N=140 | |
| Duration of follow-up | 10.2 | 14.1 |
| Median (months) (min–max) | (0.1–24.1) | (0.1–35.5) |
| Overall response rate | ||
| Investigator (95% CI) | 56.4% (48.5, 64.2) | 40.7% (32.5, 49.3) |
| BIRC (95% CI) | 46.0% (38.2, 54.0) | 35.7% (27.8, 44.2) |
| Duration of response* | ||
| Investigator (months, 95% CI) | 8.3 (6.8, 9.7) | 10.6 (7.4, 14.7) |
| BIRC (months, 95% CI) | 8.8 (6.0, 13.1) | 12.9 (9.3, 18.4) |
| Progression-free survival | ||
| Investigator (months, 95% CI) | 6.9 (5.6, 8.7) | 5.8 (5.4, 7.6) |
| BIRC (months, 95% CI) | 7.0 (5.7, 8.7) | 7.4 (5.6, 10.9) |
|Overall survival (months, 95% CI) |<>16.7 (14.8, NE) |<>15.6 (13.6, 24.2)
NE = not estimable
Study X2101: Responses assessed using RECIST 1.0
Study A2201: Responses assessed using RECIST 1.1
* Includes only patients with confirmed CR, PR
In Studies X2101 and A2201, brain metastases were seen in 60.1% and 71.4% of patients, respectively. The ORR, DOR and PFS (by BIRC assessment) for patients with brain metastases at baseline were in line with those reported for the overall population of these studies.
Non-adenocarcinoma histology
Limited information is available in ALK-positive NSCLC patients with non-adenocarcinoma histology.
Elderly
Limited efficacy data are available in elderly patients. No efficacy data are available in patients over 85 years of age.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Zykadia in all subsets of the paediatric population in lung carcinoma (small cell and non-small cell carcinoma) (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Peak plasma levels (Cmax) of ceritinib are achieved approximately 4 to 6 hours after a single oral administration in patients. Oral absorption was estimated to be ≥25% based on metabolite percentages in the faeces. The absolute bioavailability of ceritinib has not been determined.
Systemic exposure of ceritinib was increased when administered with food. Ceritinib AUCinf values were approximately 58% and 73% higher (Cmax approximately 43% and 41% higher) in healthy subjects when a single 500 mg ceritinib dose was administered with a low fat meal (containing approximately 330 kcalories and 9 grams of fat) and a high fat meal (containing approximately 1000 kcalories and 58 grams of fat), respectively, as compared with the fasted state.
In a dose optimisation study A2112 (ASCEND-8) in patients comparing Zykadia 450 mg or 600 mg daily with food (approximately 100 to 500 kcalories and 1.5 to 15 grams of fat) to 750 mg daily under fasted conditions (dose and food condition of administration initially authorised), there was no clinically meaningful difference in the systemic steady-state exposure of ceritinib for the 450 mg with food arm (N=36) compared to the 750 mg fasted arm (N=31), with only small increases in steady-state AUC (90% CI) by 4% (-13%, 24%) and Cmax (90% CI) by 3% (-14%, 22%). In contrast, the steady-state AUC (90% CI) and Cmax (90% CI) for the 600 mg with food arm (N=30) increased by 24% (3%, 49%) and 25% (4%, 49%), respectively, compared to the 750 mg fasted arm. The maximum recommended dose of Zykadia is 450 mg taken orally once daily with food (see section 4.2).
After single oral administration of ceritinib in patients, plasma exposure to ceritinib, as represented by Cmax and AUClast, increased dose-proportionally over the 50 to 750 mg dose range under fasted conditions. In contrast with single-dose data, pre-dose concentration (Cmin) after repeated daily dosing appeared to increase in a greater than dose-proportional manner.
Distribution
Binding of ceritinib to human plasma proteins in vitro is approximately 97% in a concentration independent manner, from 50 ng/ml to 10,000 ng/ml. Ceritinib also has a slight preferential distribution to red blood cells, relative to plasma, with a mean in vitro blood-to-plasma ratio of 1.35. In vitro studies suggest that ceritinib is a substrate for P-glycoprotein (P-gp), but not of breast cancer resistance protein (BCRP) or multi-resistance protein 2 (MRP2). The in vitro apparent passive permeability of ceritinib was determined to be low.
In rats, ceritinib crosses the intact blood brain barrier with a brain-to-blood exposure (AUCinf) ratio of about 15%. There are no data related to brain-to-blood exposure ratio in humans.
Biotransformation
In vitro studies demonstrated that CYP3A was the major enzyme involved in the metabolic clearance of ceritinib.
Following a single oral administration of radioactive ceritinib dose at 750 mg fasted, ceritinib was the main circulating component in human plasma. A total of 11 metabolites were found circulating in plasma at low levels with mean contribution to the radioactivity AUC of ≤2.3% for each metabolite. Main biotransformation pathways identified in healthy subjects included mono-oxygenation, O-dealkylation, and N-formylation. Secondary biotransformation pathways involving the primary biotransformation products included glucuronidation and dehydrogenation. Addition of a thiol group to O-dealkylated ceritinib was also observed.
Elimination Following single oral doses of ceritinib under fasted conditions, the geometric mean apparent plasma terminal half-life (T1⁄2) of ceritinib ranged from 31 to 41 hours in patients over the 400 to 750 mg dose range. Daily oral dosing of ceritinib results in achievement of steady-state by approximately 15 days and remains stable afterwards, with a geometric mean accumulation ratio of 6.2 after 3 weeks of daily dosing. The geometric mean apparent clearance (CL/F) of ceritinib was lower at steady-state (33.2 litres/hour) after 750 mg daily oral dosing than after a single 750 mg oral dose (88.5 litres/hour), suggesting that ceritinib demonstrates non-linear pharmacokinetics over time. The primary route of excretion of ceritinib and its metabolites is in the faeces. Recovery of unchanged ceritinib in the faeces accounts for a mean 68% of an oral dose. Only 1.3% of the administered oral dose is recovered in the urine.
Special populations
Hepatic impairment
The effect of hepatic impairment on the single-dose pharmacokinetics of ceritinib (750 mg under fasted conditions) was evaluated in subjects with mild (Child-Pugh class A; N=8), moderate (Child- Pugh class B; N=7), or severe (Child-Pugh class C; N=7) hepatic impairment and in 8 healthy subjects with normal hepatic function. The geometric mean AUC inf (unbound AUCinf) of ceritinib was increased by 18% (35%) and 2% (22%) in subjects with mild and moderate hepatic impairment, respectively, compared to subjects with normal hepatic function.
The geometric mean AUC inf (unbound AUCinf) of ceritinib was increased by 66% (108%) in subjects with severe hepatic impairment compared to subjects with normal hepatic function (see section 4.2). A dedicated pharmacokinetic study under steady-state in patients with hepatic impairment has not been conducted.
Renal impairment
A dedicated pharmacokinetic study in patients with renal impairment has not been conducted. Based on available data, ceritinib elimination via the kidney is negligible (1.3% of a single oral administered dose).
Based on a population pharmacokinetic analysis of 345 patients with mild renal impairment (CLcr 60 to <90 ml/min), 82 patients with moderate renal impairment (CLcr 30 to <60 ml/min) and 546 patients with normal renal function (≥90 ml/min), ceritinib exposures were similar in patients with mild and moderate renal impairment and normal renal function, suggesting that no dose adjustment is necessary in patients with mild to moderate renal impairment. Patients with severe renal impairment (CLcr <30 ml/min) were not included in the clinical studies of Zykadia (see section 4.2).
Effects of age, gender, and race
Population pharmacokinetic analyses showed that age, gender and race had no clinically meaningful influence on ceritinib exposure.
Cardiac electrophysiology
The potential for QT interval prolongation of ceritinib was assessed in seven clinical studies with Zykadia. Serial ECGs were collected following a single dose and at steady-state to evaluate the effect of ceritinib on the QT interval in 925 patients treated with Zykadia 750 mg once daily fasted. A central analysis of ECG data demonstrated new QTc >500 msec in 12 patients (1.3%). There were 58 patients (6.3%) with a QTc increase from baseline >60 msec. A central tendency analysis of the QTc data at average steady-state concentration from Study A2301 demonstrated that the upper bound of the 2-sided 90% CI for QTc increase from baseline was 15.3 msec at Zykadia 750 mg fasted. A pharmacokinetic analysis suggested that ceritinib causes concentration-dependent increases in QTc (see section 4.4).
Preclinical safety data
Safety pharmacology studies indicate that ceritinib is unlikely to interfere with vital functions of the respiratory and central nervous systems. In vitro data show that the IC50 for the inhibitory effect of ceritinib on the hERG potassium channel was 0.4 micromolar. An in vivo telemetry study in monkeys showed a modest QT prolongation in 1 of 4 animals after receiving the highest dose of ceritinib. ECG studies in monkeys after 4- or 13-weeks of dosing with ceritinib have not shown QT prolongation or abnormal ECGs.
The micronucleus test in TK6 cells was positive. No signs of mutagenicity or clastogenicity were observed in other in vitro and in vivo genotoxicity studies with ceritinib. Therefore, genotoxic risk is not expected in humans.
Carcinogenicity studies have not been performed with ceritinib.
Reproductive toxicology studies (i.e. embryo-foetal development studies) in pregnant rats and rabbits indicated no foetotoxicity or teratogenicity after dosing with ceritinib during organogenesis; however, maternal plasma exposure was less than that observed at the recommended human dose. Formal non-clinical studies on the potential effects of ceritinib on fertility have not been conducted.
The principal toxicity related to ceritinib administration in rats and monkeys was inflammation of the extra-hepatic bile ducts accompanied by increased neutrophil counts in the peripheral blood. Mixed cell/neutrophilic inflammation of the extra-hepatic ducts extended to the pancreas and/or duodenum at higher doses. Gastrointestinal toxicity was observed in both species characterised by body weight loss, decreased food consumption, emesis (monkey), diarrhoea and, at high doses, by histopathological lesions including erosion, mucosal inflammation and foamy macrophages in the duodenal crypts and submucosa. The liver was also affected in both species, at exposures that approximate clinical exposures at the recommended human dose, and included minimal increases in liver transaminases in a few animals and vacuolation of the intra-hepatic bile duct epithelium. Alveolar foamy macrophages (confirmed phospholipidosis) were seen in the lungs of rats, but not in monkeys, and the lymph nodes of rats and monkeys had macrophage aggregates. Target organ effects showed partial to complete recovery.
Effects on the thyroid were observed in both rat (mild increases in thyroid stimulating hormone and triiodothyronine/thyroxine T3/T4 concentrations with no microscopic correlate) and monkey (depletion of colloid in males in 4-week study, and one monkey at high dose with diffuse follicular cell hyperplasia and increased thyroid stimulating hormone in 13-week study). As these non-clinical effects were mild, variable and inconsistent, the relationship between ceritinib and thyroid gland changes in animals is unclear.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.