ZYMAR 0.3% w/v Ophthalmic solution Ref.[27534] Active ingredients: Gatifloxacin
Source: Health Products and Food Branch (CA) Revision Year: 2019
Action and clinical pharmacology
9.1 Mechanism of Action
ZYMAR is a sterile solution for topical ophthalmic use. Gatifloxacin is an 8-methoxy synthetic fluoroquinolone antibacterial agent with in vitro activity against gram-negative and grampositive, aerobic and anaerobic and clinically important atypical microorganisms.
The antibacterial action of gatifloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division (see MICROBIOLOGY).
Clinical Pharmacology
Pharmacokinetics
Ocular Administration
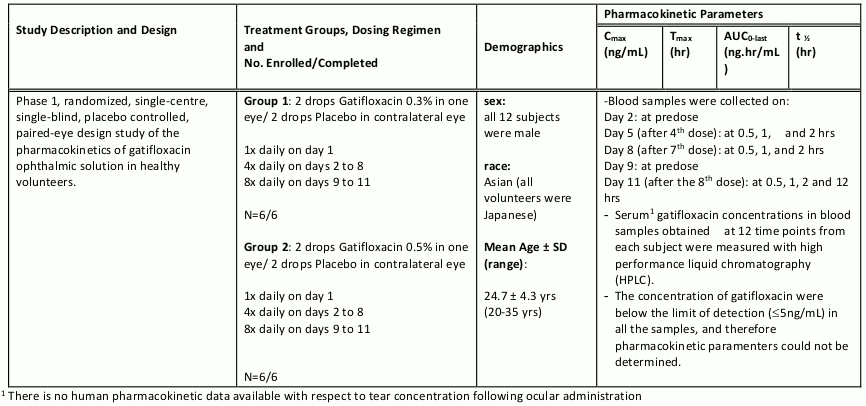
Gatifloxacin ophthalmic solutions 0.3% and 0.5% were administered to 1 eye of 6 healthy male subjects each (see PHARMACOLOGY, Human Pharmacokinetics, Table 6). At all time points, serum gatifloxacin levels were below the lower limit of quantification (5 ng/mL) in all subjects.
Pharmacokinetic parameters for ophthalmic dosing could not therefore be calculated. There is no human pharmacokinetic data available with respect to tear concentration following ocular administration.
Systemic Administration
Gatifloxacin is well absorbed from the gastrointestinal tract after oral administration and can be given without regard to food. The absolute bioavailability of gatifloxacin is 96%. Peak plasma concentrations of gatifloxacin usually occur 1-2 hours after oral dosing (see PHARMACOLOGY, Human Pharmacokinetics, Systemic Administration).
Detailed pharmacology
Preclinical Pharmacology
Pharmacokinetics
Ocular Administration
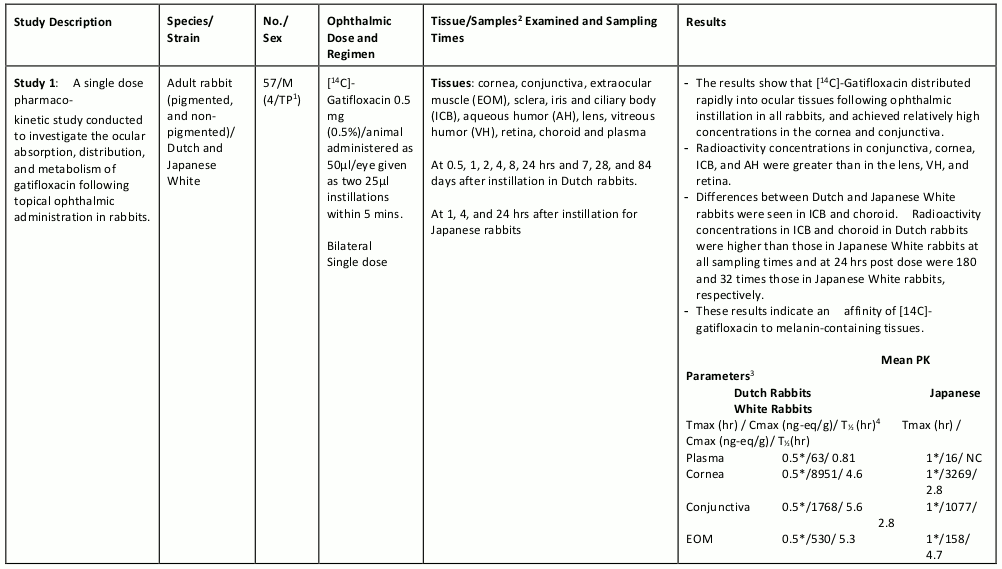
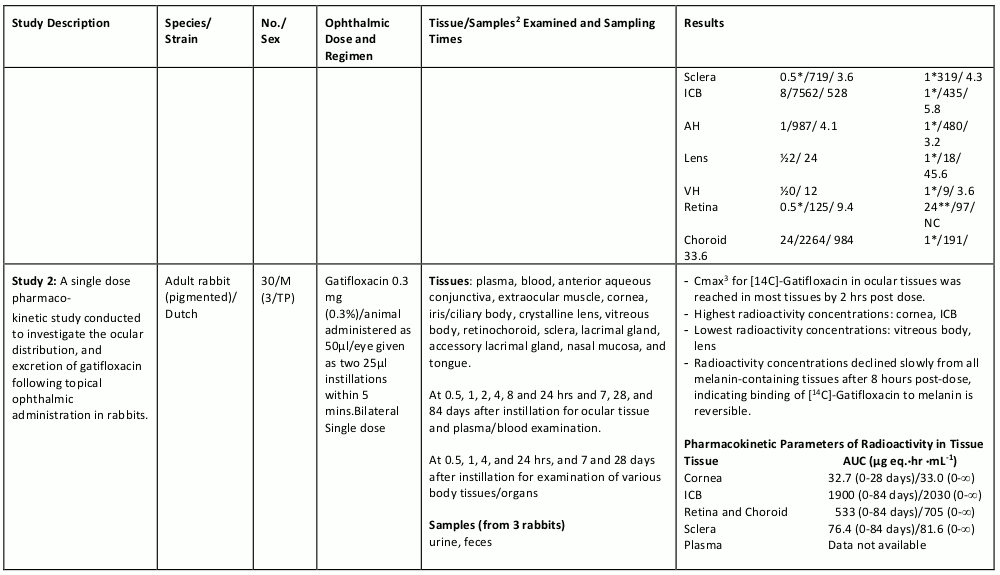
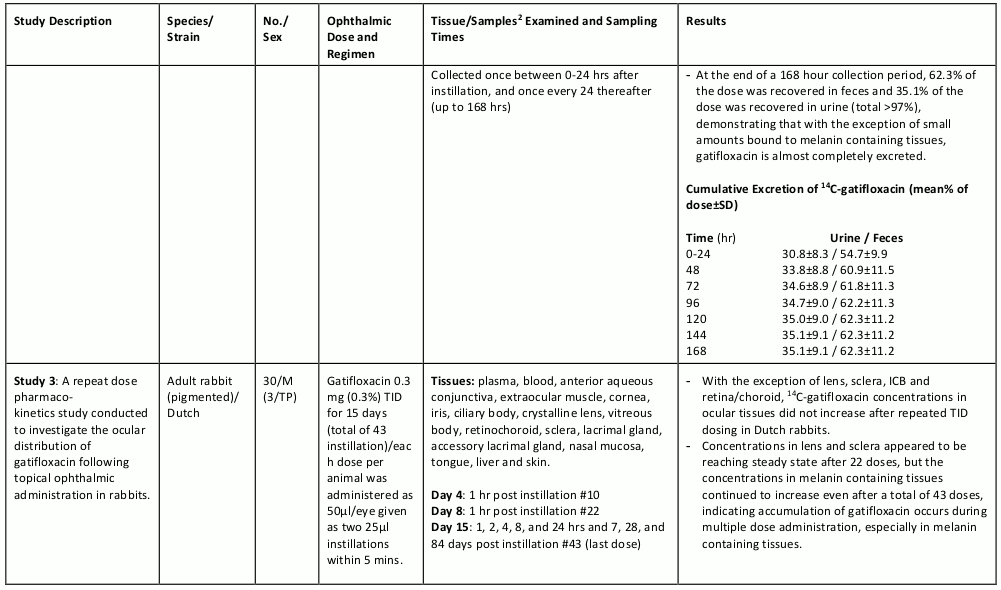
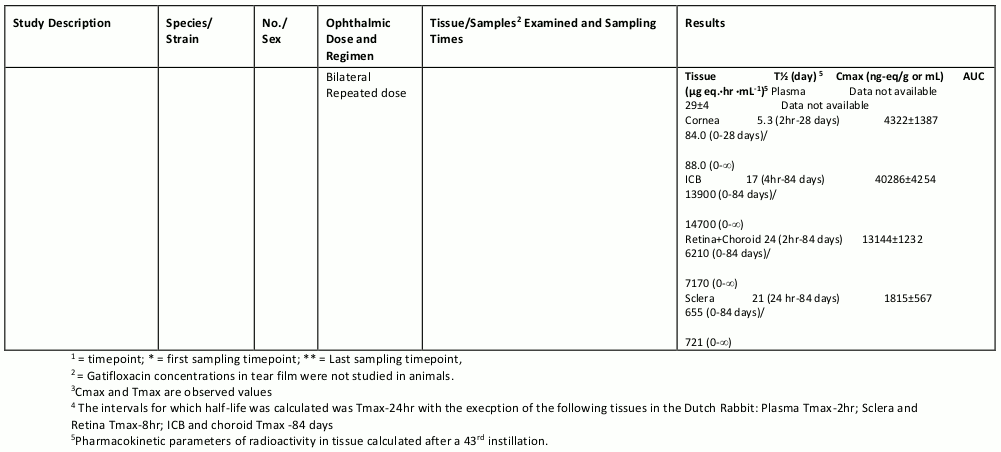
The table below summarizes the single- and multiple-dose pharmacokinetic studies conducted to study the ocular absorption, distribution, metabolism and excretion of gatifloxacin following topical ophthalmic administration.
Table 4. Preclinical Ocular Pharmacokinetic Studies:
Human Pharmacology
Pharmacokinetics
Ocular Administration
Absorption:
Systemic absorption of ZYMAR following ocular administration was investigated in 12 healthy volunteers. Below is a summary of the pharmacokinetic data from this study.
Table 5. Clinical Ocular Pharmacokinetic Studies:
Systemic Administration
Absorption:
The mean (SD) pharmacokinetic parameters of gatifloxacin after single 200 mg oral doses, single and multiple 400 mg oral doses, and single and multiple 1-hour i.v. infusions of 200 and 400 mg are listed below:
Table 6. Oral Administration:
| Cmax (μg/mL) | Tmaxa (h) | AUCb (μgh/mL) | T½ (h) | |
|---|---|---|---|---|
| 200 mg -- Healthy Volunteers | ||||
| Single dose (n=12) | 2.0 ±0.4 | 1.00 (0.50, 2.50) | 14.2 ±0.4 | -- |
| 400 mg -- Healthy Volunteers | ||||
| Single dose (n=202) | 3.8 ±1.0 | 1.00 (0.50, 6.00) | 33.0 ±6.2 | 7.8 ±1.3 |
| Multiple dose (n=18) | 4.2 ±1.3 | 1.50 (0.50, 4.00) | 34.4 ±5.7 | 7.1 ±0.6 |
| 400 mg -- Patients with Infection | ||||
| Multiple dose (n=140)c | 4.2 ±1.9 | -- | 51.3 ±20.4 | -- |
| 400 mg -- Single Dose Subjects with Renal Insufficiency | ||||
| Clcr 50-80mL/min (n=8) | 4.4 ±1.1 | 1.13 (0.75, 2.00) | 48.0 ±12.7 | 11.2 ±2.8 |
| Clcr 30-49mL/min (n=8) | 5.1 ±1.8 | 0.75 (0.50, 6.00) | 74.9 ±12.6 | 17.2 ±8.5 |
| Clcr <30mL/min (n=8) | 4.5 ±1.2 | 1.50 (0.50, 6.00) | 149.3 ±35.6 | 30.7 ±8.4 |
| Hemodialysis (n=8) | 4.7 ±1.0 | 1.50 (1.00, 3.00) | 180.3 ±34.4 | 35.7 ±7.0 |
| CAPD (n=8) | 4.7 ±1.3 | 1.75 (0.50, 3.00) | 227.0 ±60.0 | 40.3 ±8.3 |
a Median (Minimum, Maximum)
b Single dose: AUC0-_ , Multiple dose: AUC0-24
c Based on the patient population pharmacokinetic modeling, n=103 for Cmax
Cmax: Maximum serum concentration; Tmax: Time to Cmax; AUC: Area under concentration versus time curve; T1/2: Serum half-life
Table 7. Intravenous Administration:
| Cmax (μg/mL) | Tmaxa (h) | AUCb (μg·h/mL) | T½ (h) | VDss (L/kg) | |
|---|---|---|---|---|---|
| 200 mg -- Healthy Volunteers | |||||
| Single dose (n=12) | 2.2 ±0.3 | 1.00 (0.67, 1.50) | 15.9 ±2.6 | 11.1 ±4.1 | 1.9 ±0.1 |
| Multiple dose (n=8) | 2.4 ±0.4 | 1.00 (0.67, 1.00) | 16.8 ±3.6 | 12.3 ±4.6 | 2.0 ±0.3 |
| 400 mg -- Healthy Volunteers | |||||
| Single dose (n=30) | 5.5 ±1.0 | 1.00 (0.50, 1.00) | 35.1 ±6.7 | 7.4 ±1.6 | 1.5 ±0.2 |
| Multiple dose (n=5) | 4.6 ±0.6 | 1.00 (1.00, 1.00) | 35.4 ±4.6 | 13.9 ±3.9 | 1.6 ±0.5 |
a Median (Minimum, Maximum)
b Single dose: AUC0-_, Multiple dose: AUC0-24
Cmax: Maximum serum concentration; Tmax: Time to Cmax; AUC: Area under concentration versus time curve;
T1/2: Serum half-life; Vdss: Volume of distribution
Metabolism:
Following oral or i.v. administration, gatifloxacin undergoes limited biotransformation in humans with less than 1% of the dose excreted in the urine as ethylenediamine and methylethylenediamine metabolites.
In vivo studies in humans (and animals) indicate that gatifloxacin is not an enzyme inducer; therefore, gatifloxacin is unlikely to alter the metabolic elimination of itself or other coadmnistered drugs.
Distribution:
Serum protein binding of gatifloxacin is approximately 20% and is concentration independent. Following single and multiple intravenous infusions of 200 mg and 400 mg gatifloxacin, the mean volume of distribution of gatifloxacin at steady-state (Vdss) ranged from 1.5 to 2.0 L/Kg. Gatifloxacin is widely distributed throughout the body into many tissues and fluids. The distribution of gatifloxacin into tissues results in higher gatifloxacin concentrations in most target tissues than in serum.
Excretion:
Gatifloxacin is excreted as unchanged drug primarily by the kidney. More than 70% of the administered dose was recovered as unchanged drug in the urine following oral and intravenous administration, and 5% was recovered in the feces. Renal clearance is independent of dose with mean values ranging from 124 to 161 mL/min. The magnitude of this value, coupled with the significant decrease in the elimination of gatifloxacin seen with concomitant probenecid administration, indicates that gatifloxacin undergoes both glomerular filtration and tubular secretion. Gatifloxacin may also undergo minimal biliary and/or intestinal elimination, since 5% of an intravenous dose was recovered in the feces as unchanged drug.
Ophthalmic Clinical Studies
In a randomized, double-masked, multicentre clinical trial where patients, aged >1 year, were dosed for 4-6 days, ZYMAR ophthalmic solution 0.3% was superior to its vehicle on follow-up assessment (days 5-7) in patients with conjunctivitis and positive conjunctival cultures. Clinical outcomes for the trial demonstrated clinical cure of 76.9% (40/52) for the gatifloxacin treated group versus 58.3% (28/48) for the vehicle treated group on days 5-7. Microbiological outcomes for the same clinical trial demonstrated a statistically superior eradication rate for causative pathogens of 92.3% (48/52) for gatifloxacin vs. 72.3% (34/47) for vehicle on days 5-7. Please note that microbiological eradication does not always correlate with clinical cure in antiinfective trials.
Microbiology
Gatifloxacin has in vitro activity against a wide range of gram-negative and gram-positive aerobic and anaerobic microorganisms. Gatifloxacin also has in vitro activity against clinically important atypical microorganisms. The antibacterial action of gatifloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division.
The mechanism of action of fluoroquinolones including gatifloxacin is different from that of penicillins, cephalosporins, aminoglycosides, macrolides and tetracyclines. Therefore, gatifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to gatifloxacin. There is no cross-resistance between gatifloxacin and aforementioned classes of antibiotics.
Cross-resistance has been observed between systemic gatifloxacin and some other fluoroquinolones.
From in vitro synergy tests, gatifloxacin as with other fluoroquinolones is antagonistic with rifampicin against enterococci. Resistance to gatifloxacin in vitro develops slowly via multiplestep mutation. Resistance to gatifloxacin in vitro occurs at a general frequency of between 1 x 10-7 to 10-10.
Gatifloxacin has been shown to be active against most strains of the following organisms both in vitro and clinically, in conjunctival infections as described in INDICATIONS and CLINICAL USE.
Table 2. In vitro Activity of Gatifloxacin against the indicated Bacterial Isolates from Clinical Trials:
| Bacterial Species | No. of Isolates | MIC90 (μg/mL) |
|---|---|---|
| Gram-Positive Aerobic Bacteria | ||
| Staphylococcus aureus | 71 | 0.25 |
| Staphylococcus epidermidis | 94 | 2 |
| Streptococcus pneumoniae | 78 | 0.5 |
| Gram-Negative Aerobic Bacteria | ||
| Haemophilus influenzae | 93 | 0.03 |
The following in vitro data are available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of ZYMAR in treating ophthalmic infections due to the following organisms have not been established in adequate and well controlled clinical trials.
The following organisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. The following list of organisms is provided as guidance only in assessing the potential treatment of conjunctival infections.
Table 3. In vitro Activity Against Bacterial Conjunctivitis Pathogens and Ocular Pathogens:
| Organism (number of isolates) | MIC50 or MIC50 Range (µg/mL) | MIC90 or MIC90 Range (µg/mL) |
|---|---|---|
| AEROBES, GRAM-POSITIVE | ||
| Bacillus species (14) | 0.09 (9) | 0.032 - 0.120 (5) |
| Enterococcus faecalis (16) | * | 0.25 - 1.0 |
| Staphylococcus capitis (11) | * | 2 |
| Staphylococcus warneri (13) | * | 0.19-2.0 |
| Streptococcus mitis (26) | * | 0.5 |
| Streptococcus oralis (14) | * | 1 |
| Streptococcus, viridans group (24) | 0.25 (10) | 0.38 - 1.0 (14) |
| Coag Neg Staphylococcus (20) | 0.09 - 2 | * |
| AEROBES, GRAM-NEGATIVE | ||
| Moraxella catarrhalis (18) | * | 0.023 - 0.06 |
| Pseudomonas aeruginosa (39) | * | 1.95 - 32 |
| Serratia marcescens (29) | * | 0.25 - 1.0 |
* Data not available
Susceptibility Tests
There are currently no NCCLS approved standards for assessing in vitro susceptibility of conjunctival isolates to topical antibiotics, including gatifloxacin. Standardized systemic susceptibility tests may not be appropriate to predict clinical effectiveness in treating conjunctivitis.
Toxicology
Topical, Ocular Administration
Subacute and Chronic Toxicity
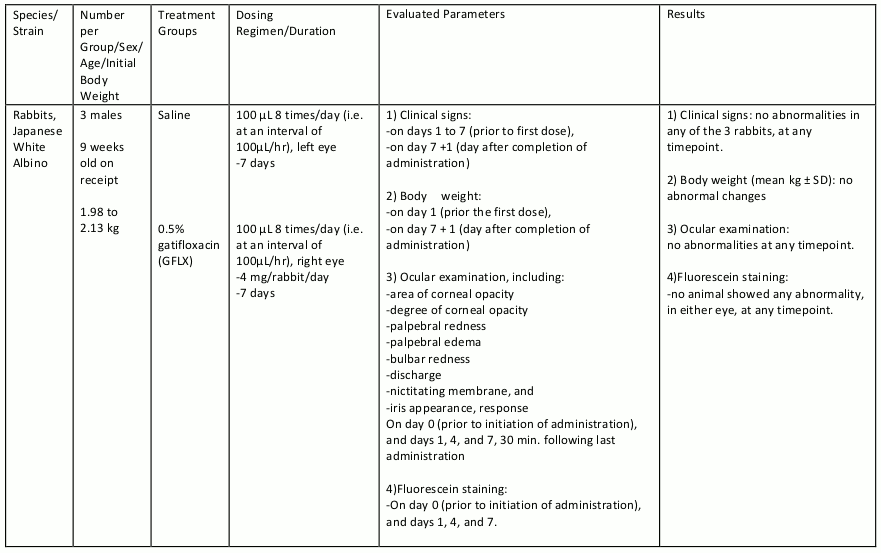
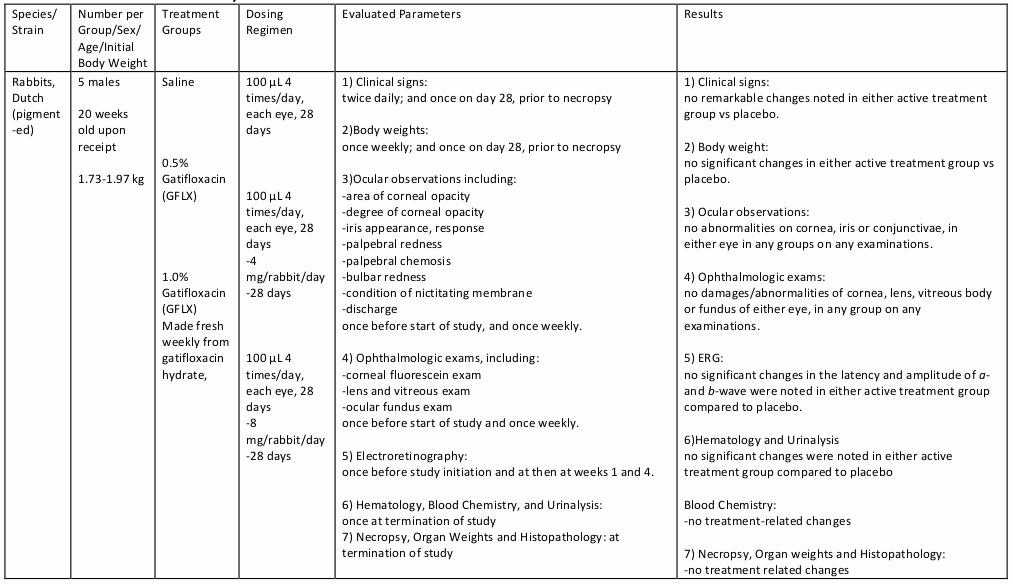
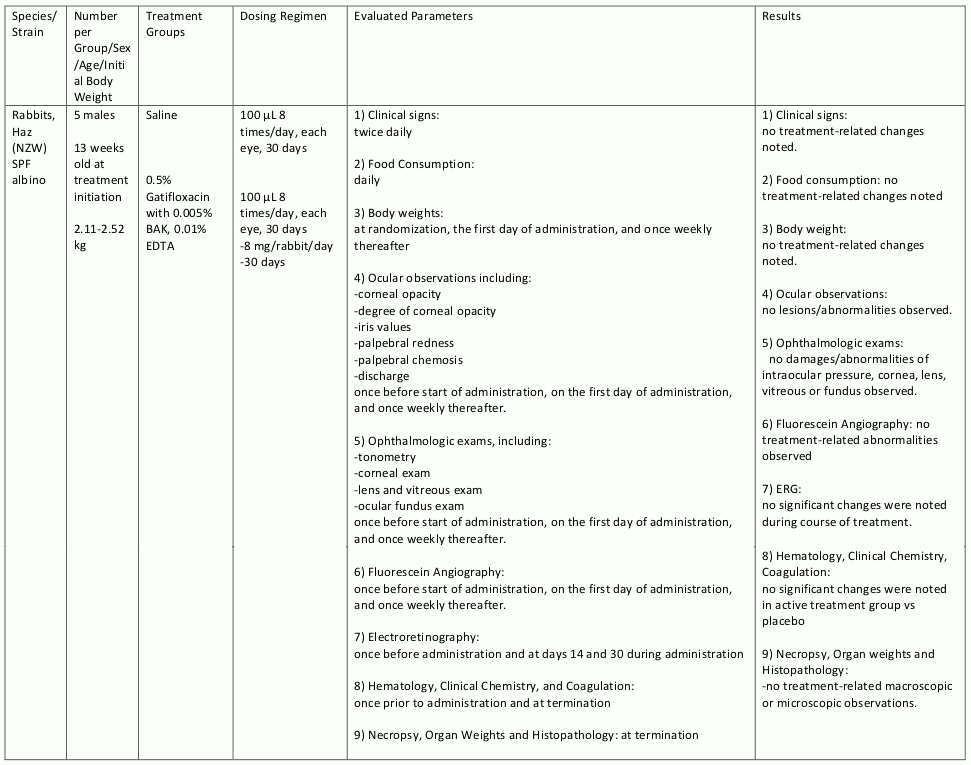
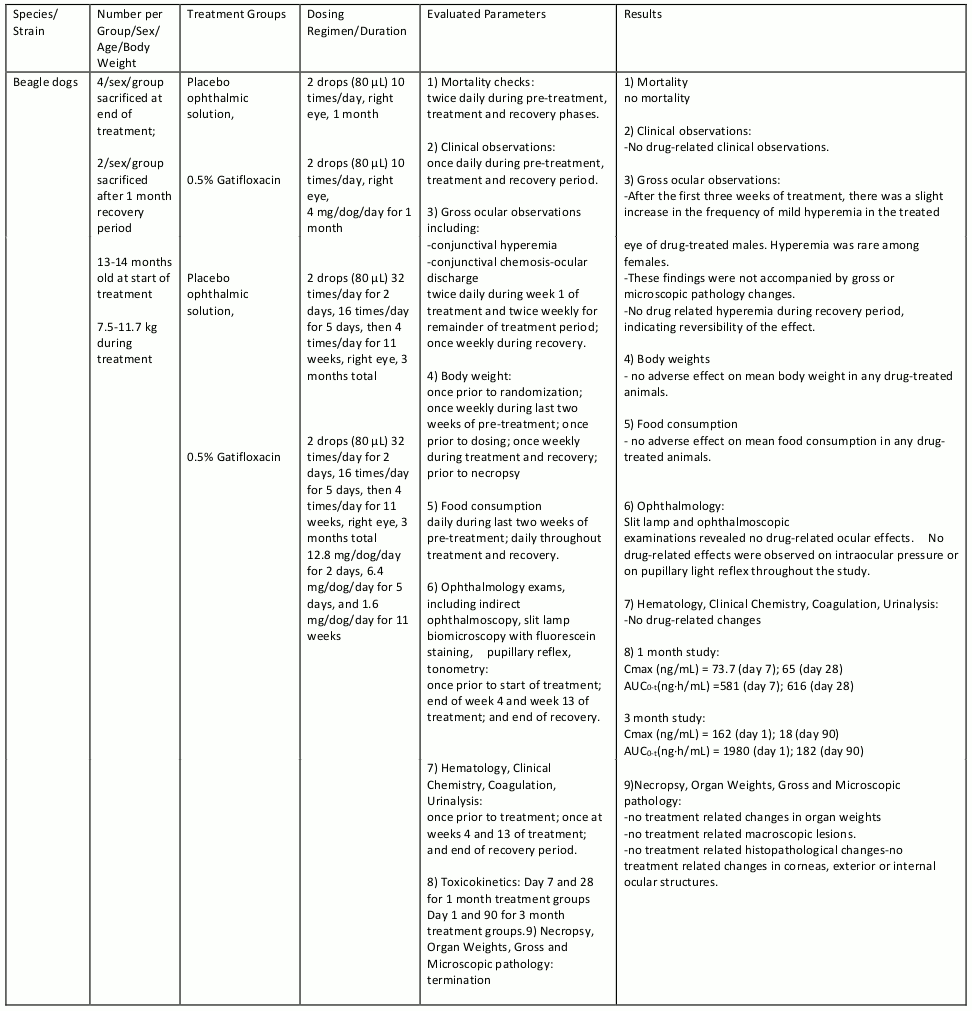
Gatifloxacin ophthalmic solution was evaluated in repeat dose ocular toxicity studies in rabbits and dogs, up to 1 month and 3 months in duration, respectively. Summaries of these studies are given in tables 8, 9, 10, and 11.
Arthrotoxic and osteotoxic potential of ZYMAR was not assessed in animals.
Table 8. Subacute Toxicity Study:
Table 9. Chronic Toxicity:
Table 10. Chronic Toxicity:
Table 11. Chronic Toxicity:
In Vitro Corneal Epithelial Wound Closure
Some quinolone antibacterials have been shown to alter corneal healing rates dose dependently in nonclinical models. In an in vitro model of wound closure in primary cultures of rabbit corneal epithelial cells, wound healing rates with gatifloxacin at 0.2 mM, 0.4 mM and 0.6 mM (75, 150, or 230 μg/mL, respectively) were 88.1, 62.8 or 33.3 percent, respectively, of the wound healing rate for untreated control cultures. Wounds in control cultures closed within 38 hours. In this assay a 5-7 mm diameter mechanical wound was made in a confluent culture of cells. Triplicate cultures were treated with each concentration of gatifloxacin, without preservatives or pharmaceutical excipients, at 37oC for 64 hours. Digital images of the wounds were taken at treatment initiation and at 13, 22, 38, 45 and 64 hrs thereafter. Wound areas were measured and relative rates of wound closure calculated (change in relative wound area per hour as a percent of the control rate).
Oral/Intravenous Administration
Acute Toxicity
In single-dose oral studies, no major adverse effects were seen in rats at doses up to 2000 mg/kg or dogs at a dose of 160 mg/kg. Single intravenous doses up to 120 mg/kg in rats and 15 mg/kg in dogs were well tolerated.
Subacute and Chronic Toxicity
In a series of repeat-dose oral studies, gatifloxacin was given for up to 6 months to rats at doses of 30, 60, 120, and 240 mg/kg/day and dogs a t doses of 6, 12, and 24 mg/kg/day. In rats, gatifloxacin was well tolerated for 6 months at a dose of 30 mg/kg daily. At 60 mg/kg/day, hepatocellular lipid droplets were observed microscopically in the liver, while at 120 mg/kg/day, and higher, similar liver changes and vacuolation of pancreatic β cells were seen. In dogs, the drug was well tolerated for 6 months at a dose of 6 mg/kg daily. At 12 mg/kg/day and higher, the primary finding was vacuolation of pancreatic β cells. In a 5 month oral monkey study (15, 30, and 60 mg/kg), drug related changes at 15 and 30 mg/kg/day were limited to vacuolation of the pancreatic β cells (only observed upon ultrastructural examination). At 60 mg/kg, in addition to the pancreatic changes, decreases in body weight and food consumption were noted. The changes observed in all of the oral studies were generally reversible upon cessation of treatment.
In 1 month intravenous studies, gatifloxacin was well tolerated in rats at doses up to 30 mg/kg daily. Doses of 90 mg/kg daily were overtly toxic, resulting in several deaths. In dogs, no drug-related changes were seen after 1 month of intravenous dosing at 7 mg/kg/day. At 15 mg/kg/day, drug-related findings were limited to emesis and salivation. Doses of 30 mg/kg daily produced numerous clinical signs, changes in clinical-pathology parameters, and a decrease in lymphocytes in the cortex of the thymus. With the exception of some minor irritation at the injection sites in rats, all of the changes observed in these studies were reversible upon cessation of treatment.
Mutagenicity
Gatifloxacin was negative in five in vivo genotoxicity studies that included oral and intravenous micronucleus tests in mice, an oral cytogenetics test in rats, and oral DNA repair tests in two strains of rats.
Gatifloxacin was evaluated as positive in three in vitro gene-mutation studies and two in vitro chromosomal-aberration studies. These findings were not unexpected; similar findings have been obtained with other quinolone antibiotics and are considered to be due to the inhibitory effects that high concentrations of these compounds have on eukaryotic cell type II DNA topoisomerase. This enzyme is related to bacterial DNA gyrase, the target at which all quinolones exert their antibiotic activity.
Carcinogenicity
There was no increase in neoplasms among B6C3F1 mice given gatifloxacin in the diet for 18 months at doses averaging 81 mg/kg/day in males and 90 mg/kg/day in females.
There was no increase in neoplasms among Fischer 344 rats given gatifloxacin in the diet for 2 years at doses averaging 47 mg/kg/day in males and 139 mg/kg/day in females. A statistically significant increase in the incidence of large granular lymphocyte (LGL) leukemia was seen in high-dose males (52%) when compared to controls (16%). Although LGL leukemia is commonly seen in the F344 rat, the incidence of this change in high-dose males slightly exceeded the historical control range (5.7 to 40.4%) established for this strain. These findings suggest that gatifloxacin may have exacerbated the onset and development of this commonly occurring neoplasm. The incidence of LGL leukemia in all of the other drug-treated groups was comparable to that in controls. There were no other neoplastic or non-neoplastic lesions observed in the study that were considered directly attributable to treatment with gatifloxacin.
Reproduction and Teratology
Animal data shows that there were no teratogenic effects observed in rats or rabbits following oral gatifloxacin doses up to 50 mg/kg/day. However, skeletal/craniofacial malformations or delayed ossification, atrial enlargement, and reduced fetal weight were observed in fetuses from rats given 150 mg/kg/day. In a perinatal/postnatal study, increased late post-implantation loss and neonatal/perinatal mortalities were observed at 200 mg/kg/day.
Special Toxicity Studies
Arthrotoxicity
Oral gatifloxacin was evaluated in a series of special toxicity studies. In juvenile rats (doses ≥600 mg/kg) and dogs (≥10 mg/kg), gatifloxacin produced arthrotoxic and osteotoxic effects similar to those seen with other quinolone antibiotics. Relevance of these findings to the clinical use of gatifloxacin ophthalmic solution is unknown.
Phototoxicity/photosensitization
There was no evidence of phototoxicity and/or photosensitization in numerous oral studies of gatifloxacin in mice and guinea pigs.
Effects on glucose/insulin/pancreatic β cells
Gatifloxacin produced reversible changes in glucose tolerance, serum insulin levels, and morphology of pancreatic β cells when given orally to rats for 7 days at a dose of 810 mg/kg/day, but not at 270 mg/kg/day. Similar changes in β cells were seen in dogs (6 months at 24 mg/kg/day) and monkeys (5 months at 60 mg/kg/day) given gatifloxacin orally.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.