ZYNQUISTA Film-coated tablet Ref.[10997] Active ingredients: Sotagliflozin
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Guidehouse Germany GmbH, Albrechtstr. 10c, 10117 Berlin, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, sodium-glucose co-transporter 2 (SGLT2) inhibitors
ATC code: A10BK06
Mechanism of action
Sotagliflozin is a dual inhibitor of sodium glucose cotransporter type 1 (SGLT1) and SGLT2. Local intestinal inhibition of SGLT1, the major transporter for glucose absorption, delays and reduces glucose absorption in the proximal intestine, resulting in a blunting and delay of postprandial hyperglycaemia. SGLT2 is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. By inhibiting SGLT2, sotagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Pharmacodynamic effects
Urinary glucose excretion
In a 12-week dose ranging study, consistent with SGLT2 inhibition, 24-h placebo corrected change from baseline Urinary Glucose Excretion (UGE) increased by 57.7 grams (p <0.001) and 70.5 grams (p <0.001) in patients with type 1 diabetes taking 200 mg and 400 mg sotagliflozin respectively, which is consistent with SGLT2 inhibition.
Post prandial glucose reduction
In a 12 week dose ranging study, consistent with SGLT1 inhibition, placebo corrected change from baseline 2-hour post prandial glucose (PPG), measured after a standardised mixed meal, was reduced by 1.52 mmol/L (p=0.15) and 2.73 mmol/L (p=0.006) in patients taking 200 mg and 400 mg sotagliflozin respectively, which is consistent with SGLT1 inhibition.
Clinical efficacy and safety
The efficacy and safety of sotagliflozin in patients with type 1 diabetes not adequately controlled on their current insulin therapy was evaluated in three double-blind, placebo-controlled studies. In InTandem1 (study 1) and in InTandem2 (study 2) sotagliflozin was used as add on to optimised insulin and in InTandem3 (Study 3) sotagliflozin was used as add on to any existing insulin regimen in patients not on HbA1c goal.
Study 1 and study 2
On a background of optimised insulin, the efficacy and safety of sotagliflozin 200 mg or 400 mg once daily versus insulin alone were evaluated in two double-blind, placebo controlled studies (study 1 and 2) performed in 1575 patients with type 1 diabetes on insulin pump or multiple daily injection therapy. Each study was of 52 weeks duration, with primary and key secondary endpoints at 24 weeks. Beginning 6 weeks prior to randomization, insulin dose was adjusted (optimised) to achieve the following glycaemic goals fasting/preprandial, self-monitored blood glucose (SMBG) 4.4-7.2 mmol/L and 2-hour/peak postprandial SMBG glucose <10 mmol/L.
Patients were then maintained on optimised insulin and randomized to sotagliflozin 200 mg, sotagliflozin 400 mg or insulin alone. For the first meal on Day 1, patients were instructed to decrease their calculated (or usual) mealtime carbohydrate bolus insulin by 30%. Insulin optimisation was continued throughout the study.
In study 1, a total of 793 patients entered the study. The mean age of patients was 46 years, 8.1% were 65 years or older. Mean duration of diabetes was 24.4 years, 60% of patients were using insulin pump and 40% were using multiple daily injections. In the study 48% were male and 92% were White and 84% of randomised patients completed the study. The mean eGFR was 87 mL/min/1.73 m² and 5.7% of patients had eGFR between 45 and 60 mL/min/1.73 m². The mean BMI was 30 kg/m² and 23% of patients had SBP ≥ 130 mmHg. At screening the HbA1c was 8.21%, 8.26% and 8.20% for insulin, insulin+sotagliflozin 200 mg and insulin+sotagliflozin 400 mg.
In study 2, a total of 782 patients entered the study. The mean age of patients was 41 years, 4.2% were 65 years or older. Mean duration of diabetes was 18 years, 26% of patients were using insulin pump and 74% were using multiple daily injections. In the study 52% were male and 96.2% were White and 87% of randomised patients completed the study. The mean eGFR was 92 mL/min/1.73 m² and 3.3% had eGFR between 45 and 60 mL/min/1.73 m². The mean BMI was 28 kg/m² and 32% of patients had SBP ≥ 130 mmHg. At screening the HbA1c was 8.42%, 8.35% and 8.38% for insulin, insulin+sotagliflozin 200 mg and insulin+sotagliflozin 400 mg.
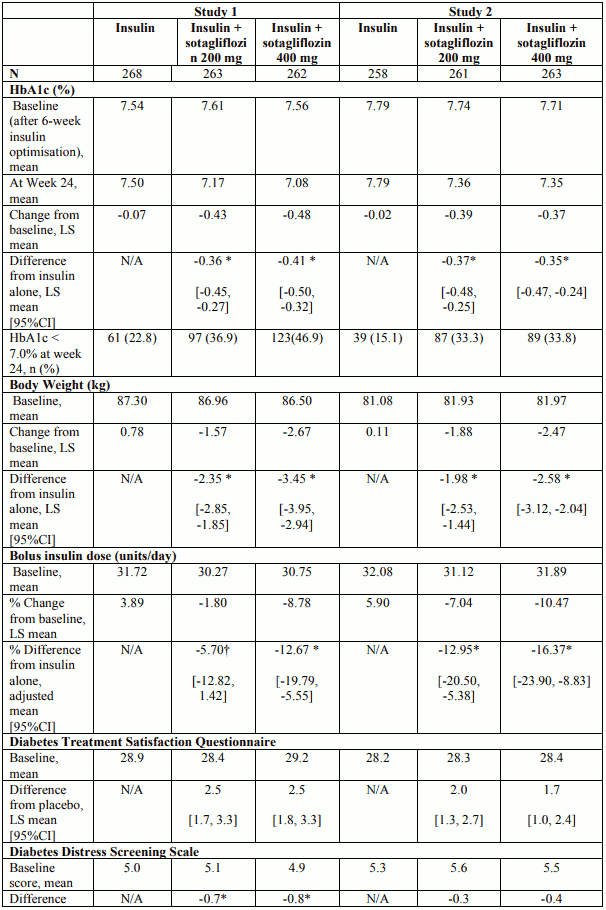
At week 24, treatment with 200 mg or 400 mg sotagliflozin provided statistically significant reductions in HbA1c (p-value <0.001) compared to insulin alone. Treatment with sotagliflozin also resulted in reduction in body weight and FPG compared with insulin alone (see Table 4).
Main results for insulin dose and Diabetes Treatment Satisfaction Questionnaire and Diabetes Distress Screening Scale are presented in Table 4.
Table 4. Results at 24-week trial with sotagliflozin in patients with type 1 diabetes mellitus inadequately controlled on insulin (Study 1 – Study 2):
No differences in HbA1c decrease could be detected across subgroups including age, gender, race, geographic region, baseline BMI, age at diagnosis, baseline HbA1c, eGFR, duration of disease and insulin delivery method.
In studies 1 and 2 combined, patient 24-week completion rates were 89.5% among insulin alone patients, and 91.4% and 90.7% among patients receiving 200 mg and 400 mg of sotagliflozin, respectively. The 52-week completion rates were 84.2%, 86.6%, and 85.3% respectively.
Efficacy over a 52-week period
At the end of 24 weeks the reduction in HbA1c was -0.36% and -0.38% and at 52 weeks it was -0.23% and -0.32% with sotagliflozin 200mg and 400 mg, respectively. The proportion of patients with A1C <7.0% at 24 weeks was 19.0% for placebo, 35.1% for sotagliflozin 200mg, 40.4% for sotagliflozin 400mg and at 52 weeks was 18.3%, 28.6% and 31.6% for placebo, sotagliflozin 200 mg and 400 mg respectively.
At the end of 52 weeks the reduction in body weight, mean daily bolus insulin dose, FPG were sustained compared to insulin alone.
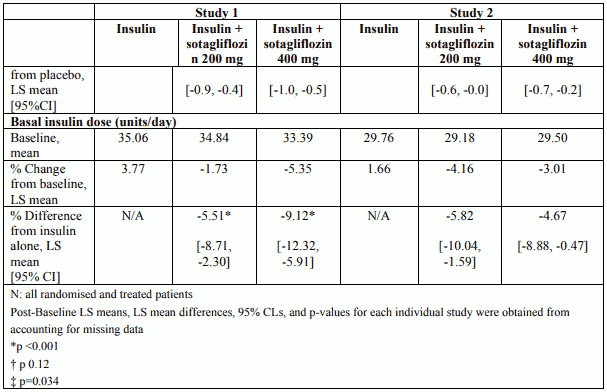
CGM substudy: 2-hr PPG and time in range
From study 1 and study 2, 278 subjects participated in a blinded continuous glucose monitoring (CGM) sub-study (see Table 5).
Table 5. Results of CGM sub study at week 24 (Pooled data, study 1 and study 2):
Study 3
InTandem 3 (study 3) was a 24-week duration study performed on a background of existing insulin regimen in type 1 diabetes patients with screening HbA1c ≥7.0% to ≤11.0%, to evaluate efficacy and safety of sotagliflozin 400 mg once daily versus insulin alone.
For the first meal on Day 1, patients were instructed to decrease their calculated (or usual) mealtime carbohydrate bolus insulin by 30%.
The mean age of patients was 43 years, 7.2% were 65 years or older. Mean duration of diabetes was 20 years, 39% of patients were using insulin pump and 61 % were using non-pump insulin therapy. In the study 50% were male and 88 % were White and 87% of randomised patients completed the study. The mean eGFR was 92 mL/min/1.73 m² and 5 % had eGFR between 45 and 60 mL/min/1.73 m². The mean BMI was 28 kg/m² and 29% had SBP ≥130 mmHg.
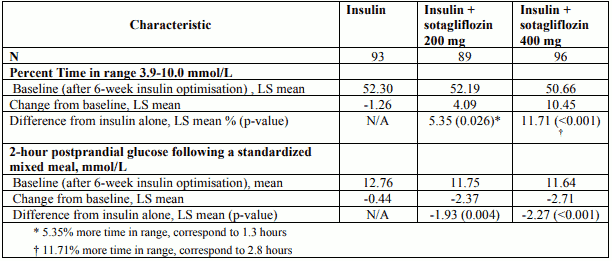
At week 24, treatment with 400 mg sotagliflozin before the first meal of the day resulted in statistically significant more patients achieving the net benefit primary endpoint (proportion of patients with HbA1c <7.0% at week 24 and no episode of severe hypoglycaemia, and no episode of DKA from randomisation to Week 24) compared with insulin alone (28.6% versus 15.2%) (p-value<0.001) and provided statistically significant mean reductions in HbA1c (p-value <0.001). Treatment with sotagliflozin also resulted in reduction in body weight and bolus insulin dose compared with insulin alone (see Table 6). Treatment with sotagliflozin also resulted in reduction in body weight and systolic blood pressure (in patients with baselines SBP ≥130 mmHg) compared with insulin alone (see Table 6). Main results regarding insulin dose are presented in Table 6.
Table 6. Efficacy results of a 24 week placebo-controlled study of sotagliflozin as add-on to insulin therapy in patients not at HbA1c goal (Study 3):
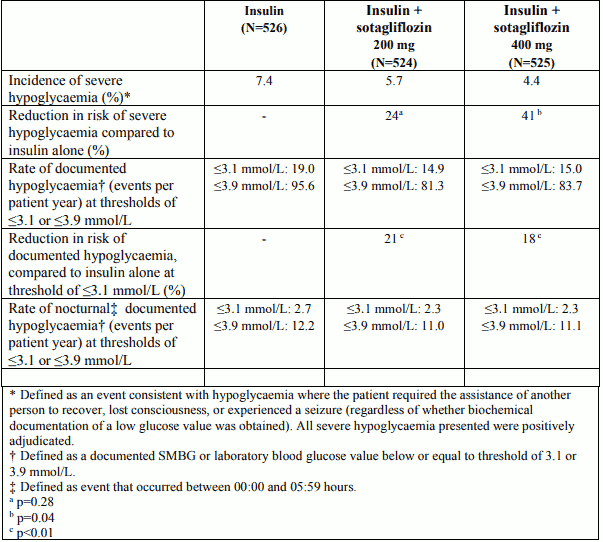
Hypoglycaemia
The incidence of severe hypoglycaemia and rates of documented hypoglycaemia (overall and nocturnal) were lower on sotagliflozin compared to insulin alone in the 52-week studies, as shown in Table 7.
Table 7. Incidence of severe hypoglycaemia and rates of documented, (overall and nocturnal) hypoglycaemic events in the pool of two 52-week placebo-controlled clinical studies:
In study 3 at 24 weeks incidences of severe hypoglycaemia were 2.4% and 3.0% on placebo and sotagliflozin 400 mg, respectively and the reduction in the rate of hypoglycaemic events at 24 weeks (blood glucose ≤3.1 mmol/L) for sotagliflozin 400 mg was 22% (p<0.001) compared to insulin alone.
Patients with renal impairment
In the 3 Phase 3 randomized clinical studies in patients with type 1 diabetes, patients with eGFR <45 mL/min/1.73 m² were excluded, 79 patients exposed to sotagliflozin had an eGFR <60 mL/min/1.73 m² and 841 patients had an eGFR ≥60 to ≤90 mL/min/1.73 m². The HbA1c reduction observed in patients with eGFR ≥60 to <90 mL/min/1.73 m² was comparable to the HbA1c reduction observed in patients with eGFR ≥90 mL/min/1.73 m². In patients with eGFR <60 mL/min/1.73 m² a numerical HbA1c reduction was observed. No overall differences in safety were observed with sotagliflozin treatment as compared to insulin alone in subjects with eGFR between 45 and 60 mL/min/1.73 m².
Fasting plasma glucose
In a pre-specified pooled analysis of study 1 and study 2, treatment with sotagliflozin as adjunct to insulin resulted in LS mean changes from baseline in FPG of -0.56 mmol/l for sotagliflozin 200 mg and -0.87 mmol/l for sotagliflozin 400 mg compared to insulin alone (0.32 mmol/l) at week 24. In study 3 there was a significant reduction in FPG of 0.79 mmol/L (p<0.001) with sotagliflozin 400 mg at 24 weeks compared to insulin alone.
Blood pressure
In a pre-specified pooled analysis of study 1 and study 2, treatment with sotagliflozin as adjunct to insulin resulted in a reduction of SBP (-0.6 mmHg for placebo, -2.6 mmHg for sotagliflozin 200 mg and -4.1 mmHg for sotagliflozin 400 mg) at week 12. Pooled analysis of change in SBP in patients with baseline SBP ≥130 mmHg showed greater reduction in SBP at week 12 (-5.4 mmHg for placebo, -9.0 mmHg for sotagliflozin 200 mg and -10.7 mmHg for sotagliflozin 400 mg).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Zynquista in one or more subsets of the paediatric population in type 1 diabetes mellitus (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The pharmacokinetics (PK) of sotagliflozin has been characterized in healthy subjects and in diabetic patients. No clinically relevant differences were noted between the two populations.
Absorption
The median Tmax ranged from 1.25 to 3 hours, over a single-dose range of 400 to 2000 mg. Following administration of multiple doses (400 and 800 mg dose), the median Tmax values ranged from 2.5 to 4 hours.
The fraction of drug absorbed following administration of a single dose of [14C]-sotagliflozin was estimated to be at least 71%, based on the detected percentage of dose of radioactivity in the urine and for the metabolites of sotagliflozin in the faeces.
When sotagliflozin tablets were administered with a high-caloric breakfast, plasma exposure to sotagliflozin as measured by Cmax and AUC0-inf was about 2.5- and 1.5-fold higher, respectively, compared to fasted condition.
Distribution
Both sotagliflozin and its major human metabolite 3-O-glucuronide (M19), exhibited high binding to human plasma proteins in vitro (fraction unbound approx. 2%) which was not dependent on the concentration of sotagliflozin and M19. In clinical studies the high protein binding was confirmed and was not influenced by reduced renal or hepatic function. The apparent volume of distribution of sotagliflozin following administration of a single 400 mg oral dose of [14C]-sotagliflozin was found to be very high with a mean value of 9392 L.
Biotransformation
In healthy subjects following the administration of a single dose of 400 mg [14C]-sotagliflozin indicated that sotagliflozin was extensively metabolized predominantly to M19 which represented 94% of the radioactivity in plasma.
The primary route of metabolism of sotagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases primarly by UGT1A9, and to a much lesser extent by UGT1A1 and UGT2B7 as well as oxidation, by CYP3A4.
When sotagliflozin is incubated with UGT1A9, M19 was the main conjugate observed. No acyl glucuronides of sotagliflozin were identified.
In in vitro studies, sotagliflozin did not inhibit CYP 1A2, 2C9, 2C19, 2D6, or 3A4, nor induced CYP 1A2, 2B6, or 3A4. Sotagliflozin and M19 have no significant potential to inhibit OCT1, OCT2, OAT1, OAT3, OATP1B1 and OATP1B3.
M19 is an inducer and inhibitor of CYP3A4 and an inhibitor of CYP2D6. In vitro sotagliflozin was shown to have inhibitory effects on P-gp and breast cancer resistance protein (BCRP). M19 demonstrated inhibitory effects against OATP1B1/B3 and MRP2 in-vitro.
Elimination / excretion
Following the administration of a single dose of 400 mg [14C]-sotagliflozin, showed 57% and 37% of the radioactivity were excreted in the urine and faeces, respectively. These results indicate that the main route of elimination of drug related material was renal. The predominant metabolite detected in urine was M19, representing a mean of 33% of the administered radioactive dose. Unchanged [14C]-sotagliflozin was the predominant radioactive peak detected in faecal extracts representing a mean of 23% of the total administered radioactive dose. In healthy volunteers, mean apparent total body clearance (CL/F) of sotagliflozin ranged from 261 to 374 L/hr. The CL/F estimated using a population PK, which mostly evaluated T1DM patients, was 239 L/hr. Mean terminal T1/2 ranged from 21 to 35 hours for sotagliflozin and from 19 to 26 hours for M19.
Linearity / non-linearity
The PK of sotagliflozin appeared to be dose proportional in the therapeutic dose range of 200 mg to 400 mg QD.
Special populations
Renal impairment
Exposure of sotagliflozin was evaluated in a dedicated study of subjects with mild (creatinine clearance [CLcr]: 60 to less than 90 mL/min) and moderate (CLcr: 30 to less than 60 mL/min) renal impairment and with normal renal function. In subjects with renal impairment, exposure to sotagliflozin following a single dose of 400 mg was approximately 1.7 fold higher in subjects with mild and up to 2.7 fold higher in subjects with moderate renal impairment compared to subjects with normal renal function.
The apparent clearance of sotagliflozin decreases with decreasing renal function. A population PK model integrating data from renally impaired patients and healthy subjects estimated for subjects with CKD Stage II (eGFR ≥60 and <90 mL/min/1.73 m²) and CKD Stage IIIa (eGFR ≥45 and <60 mL/min/1.73 m²) that sotagliflozin exposures were 1.5 fold higher compared to subjects with normal renal function. For subjects with CKD Stage IIIb (eGFR ≥30 and <45 mL/min/1.73 m²) and CKD Stage IV (eGFR ≥15 and <30 mL/min/1.73 m²) sotagliflozin exposures were 1.95 and 2.25 fold higher compared to subjects with normal renal function.
Hepatic impairment
In a study with subjects with reduced hepatic function, AUC of sotagliflozin was not increased in mild (Child Pugh A) hepatic impaired subjects, but was increased by ~3 fold in moderate (Child Pugh B) and ~6 fold in severe (Child Pugh C) hepatic impaired subjects. No dose adjustment is needed in patients with mild hepatic impairment.
Elderly
Based on a population PK analysis, age had no clinically meaningful effect on the pharmacokinetics of sotagliflozin.
Body weight
Based on a population PK analysis, sotagliflozin exposure was found to decrease with increased body weight. Consequently, low-weight patients may have somewhat increased exposure and patients with high weight somewhat decreased exposure. However, the differences in exposure were not considered clinically meaningful and therefore no dose adjustment is required based on weight.
Gender and race
Based on a population PK analysis gender and race had no clinically meaningful effect on the PK of sotagliflozin.
Paediatric patients
No data are available.
5.3. Preclinical safety data
In a rat carcinogenicity study, a statistically significant increase in thyroid follicular cell carcinoma was observed in males at 75 mg/kg/day, approx. 14 times the MRHD, the highest dose evaluated. In a repeat-dose study evaluating potential mechanisms responsible for the increase incidence of thyroid carcinoma observed in the rat carcinogenicity study, it was concluded that the increase was associated with a sotagliflozin-related increase in thyroid stimulating hormone (TSH). In the rat, TSH was considered the primary carcinogen with sotagliflozin functioning as a secondary carcinogen. These changes were not considered relevant for humans as TSH is not carcinogenic in humans.
Sotagliflozin was not mutagenic or clastogenic.
In a fertility study in rats, sotagliflozin had no effect on reproductive performance, fertility, and embryo/fetal viability.
In a juvenile toxicology study in rats, renal changes were observed when sotagliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy. Exposure was approximately 5 times (males) and 11 times (females) the clinical exposure at the Maximum Recommended Human Dose (MRHD) and caused reversible renal tubular dilation.
In embryo-fetal development studies in rats and rabbits, sotagliflozin was orally administered at doses up to 350 mg/kg in rats and 200 mg/kg in rabbits. In the rat study, embryo-lethality, effects on fetal growth along with cardiovascular and skeletal abnormalities were observed at an exposure multiple of 158-times the human exposure at 400 mg/day. The adverse effects on embryo-fetal development at 350 mg/kg/day were associated with maternal toxicity (body weight loss/decreased body weight gain and decreased food consumption during gestation day (GD) 6 to 8). Exposure at the rat no-observedeffect level was 40-times the exposure at the MRHD. No developmental toxicity was observed at doses up to 200 mg/kg/day in the rabbit, which was up to 9-times the human exposure at the MRHD.
In a pre-/post-natal development study, no sotagliflozin-related adverse effects in pregnant and lactating females and offspring development were observed in rats In a study evaluating the potential effects of sotagliflozin on the development of juvenile rats, no sotagliflozin-related toxicity was observed following the administration of oral doses up to approximately 18 and 31 fold that of the MRHD (400 mg/day) for males and females, respectively.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.