ZYNTEGLO Dispersion for infusion Ref.[50056] Active ingredients: Betibeglogene autotemcel
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: bluebird bio (Netherlands) B.V., Stadsplateau 7, WTC Utrecht, 3521AZ Utrecht, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other haematological agents
ATC code: B06AX02
Mechanism of action
Zynteglo adds functional copies of a modified β-globin gene into the patients' HSCs through transduction of autologous CD34+ cells with BB305 LVV, thereby addressing the underlying genetic cause of the disease. After Zynteglo infusion, transduced CD34+ HSCs engraft in the bone marrow and differentiate to produce RBCs containing biologically active βA-T87Q-globin (a modified β-globin protein) that will combine with α-globin to produce functional Hb containing βA-T87Q-globin (HbAT87Q). βA-T87Q-globin can be quantified relative to other globin species in peripheral blood using high performance liquid chromatography. βA-T87Q-globin expression is designed to correct the β/α-globin imbalance in erythroid cells of patients with TDT and has the potential to increase total Hb to normal levels and eliminate dependence on chronic RBC transfusions. Following successful engraftment and achievement of transfusion independence, the effects of the product are expected to be life-long.
Pharmacodynamic effects
All patients with TDT with a non-β0/β0 genotype who received Zynteglo with at least 3 months of follow-up produced HbAT87Q (N=10, HGB-204; N=4, HGB-205; N=15, HGB-207; N=3, HGB-212). For patients with at least 6 months of follow-up, HbAT87Q generally increased steadily after Zynteglo infusion and stabilised by approximately Month 6. Patients had a Month 6 median (min, max) HbAT87Q f 4.901 (1.03, 9.59) g/dL in the Phase ½ studies (N=14, HGB-204 and HGB-205) and 9.409 (3.35, 10.60) g/dL in ongoing Phase 3 studies (N=16, HGB-207 and HGB-212).
HbAT87Q remained generally stable through Month 24 with a median (min, max) of 6.444 (1.10, 10.13) g/dL in the completed Phase ½ studies (N=14, HGB-204 and HGB-205) and 8.766 (0.89, 11.40) g/dL in the ongoing Phase 3 studies (N=3, HGB-207). HbAT87Q continued to be stable at last follow-up through Month 60, demonstrating stable integration of the βA-T87Q-globin gene into long-term HSCs and stable expression of the βA-T87Q-globin gene in cells of the erythroid lineage.
Clinical efficacy
Efficacy was based on 32 adult and adolescent patients with TDT and a non-β0/β0 genotype treated with Zynteglo (N=10, HGB-204; N=4, HGB-205; N=15, HGB-207; N=3, HGB-212) (see Table 4). A few patients have been included in the clinical studies with genotypes characterised by low endogenous β-globin production phenotypically similar to patients with a β0 /β0 genotype, such as patients homozygous for IVS-I-110 or IVS-I-5.
Table 4 Baseline characteristics for non-β0/β0 patients with TDT ≥12 years of age treated with Zynteglo (Studies HGB-204, HGB-205, HGB-207, HGB-212, LTF-303):
| Study | Total number of patients (young adults/ adolescents) | Age (years) median (min, max) | Pre-enrolment transfusion volumes (mL/kg/year) median (min, max) | Pre-enrolment transfusion frequency (number/year) median (min, max) |
|---|---|---|---|---|
| HGB-205 | 4 (2) | young adults/ adolescents* | 181.85 (138.8, 197.3) | 12.50 (10.5, 13.0) |
| HGB-204 | 10 (2) | 19.5 (16, 34) | 151.28 (140.0, 234.5) | 13.75 (10.0, 16.5) |
| HGB-207 | 15 (6) | 20.0 (12, 34) | 192.92 (152.3, 251.3) | 17.50 (11.5, 37.0) |
| HGB-212 | 3 (1) | adults/ adolescents* | 175.51 (170.7, 209.6) | 21.50 (17.5, 39.5) |
* Age range is not provided to protect patient identity.
Transfusion-dependent β-thalassaemia (TDT)
Patients were considered to be transfusion-dependent if they had a history of transfusions of at least 100 mL/kg/year of RBCs or with ≥8 transfusions of RBCs per year in the 2 years preceding enrolment. In the clinical studies, patients received a median (min, max) RBC transfusion volume of 175.74 (138.8, 251.3) mL/kg/year and a median (min, max) number of 14.75 (10.0, 39.5) RBC transfusions per year.
Adolescents were excluded from Phase 3 studies if they had a known and available HLA-matched related HSC donor. The median (min, max) age in the studies was 19.0 (12, 34) years, 56.3% were females, 59.4% were Asian, and 40.6% White/Caucasian. All patients had a Karnofsky/Lansky performance score ≥80 and the majority (18/32, 56.3%) had a performance score of 100 at baseline. Cardiac T2* at baseline was >20 msec. The median (min, max) serum ferritin at baseline was 3778.7 (784, 22517) pmol/L and median (min, max) liver iron concentration was 6.75 (1.0, 41.0) mg/g (N=10, HGB-204; N=4, HGB-205; N=15, HGB-207; N=3, HGB-212).
Mobilisation and apheresis
All patients were administered G-CSF and plerixafor to mobilise stem cells prior to the apheresis procedure. The planned dose of G-CSF was 10 µg/kg/day in patients with a spleen, and 5 µg/kg/day in patients without a spleen, given on Days 1 through 5 of mobilisation in the morning. The planned dose of plerixafor was 0.24 mg/kg/day, given on Days 4 and 5 of mobilisation in the evening. If a third day of collection was needed, plerixafor and G-CSF dosing was extended to Day 6. The dose of G-CSF was decreased by half if white blood cell (WBC) count was >100 × 109/L prior to the day of apheresis. For most patients, the minimum number of CD34+ cells to manufacture Zynteglo was collected with 1 cycle of mobilisation and apheresis.
Pre-treatment conditioning
All patients received full myeloablative conditioning with busulfan prior to treatment with Zynteglo. The planned dose of busulfan was 3.2 mg/kg/day for patients ≥18 years as a 3-hour IV infusion daily for 4 days with a recommended target AUC0-24h of 3800-4500 µM*min. The planned dose of busulfan was 0.8 mg/kg for patients 12-17 years of age as a 2-hour IV infusion every 6 hours for a total of 16 doses with a recommended target of AUC0-6h of 950-1125 µM*min. The busulfan SmPC was used for information on appropriate method for determination of patient weight-based dosing. Busulfan dose adjustments were made as needed based on pharmacokinetic monitoring.
The median (min, max) busulfan dose was 3.50 (2.5, 5.0) mg/kg/day (N=32). AUC0-24h was measured during Day 1 and informed the dose for Day 3; the median (min, max) estimated daily AUC was 4394.5 (3030, 9087) μM*min (N=32). All patients with non-β0/β0 genotypes received anti-seizure prophylaxis with agents other than phenytoin prior to initiating busulfan. Phenytoin was not used for anti-seizure prophylaxis because of its well understood induction of glutathione-S-transferase and cytochrome P450 and resultant increased clearance of busulfan, and because of the widespread availability of effective anti-seizure medications that do not affect busulfan metabolism.
In HGB-207 and HGB-212 prophylaxis for VOD/hepatic sinusoidal obstruction syndrome was required per institutional practice with ursodeoxycholic acid or defibrotide.
Zynteglo administration
All patients were administered Zynteglo with a median (min, max) dose of 7.80 × 106 (5.0, 19.4) CD34+ cells/kg as an intravenous infusion (N=32).
After Zynteglo administration
A total of 31.1% of patients (14/45; HGB-204, HGB-205, HGB-207, HGB-212) received G-CSF within 21 days after Zynteglo infusion. However, G-CSF use was not recommended for 21 days after Zynteglo infusion in Phase 3 studies.
Studies HGB-204 and HGB-205
HGB-204 and HGB-205 were Phase ½ open-label, single-arm 24-month studies that included 22 patients with TDT treated with Zynteglo (N=18, HGB-204; N=4, HGB-205), of whom 14 had a non-β0/β0 genotype (N=10, HGB-204; N=4, HGB-205) and 8 had a β0 /β0 genotype in HGB-204. All patients completed HGB-204 and HGB-205 and enrolled for long-term follow-up in the LTF-303 study. The median (min, max) duration of follow-up of patients with a non-β0/β0 genotype was 44.63 (35.8, 61.3) months. All patients remain alive at last follow-up.
The primary endpoint was transfusion independence (TI) by Month 24, defined as a weighted average Hb ≥9 g/dL without any RBC transfusions for a continuous period of ≥12 months at any time during the study after infusion of Zynteglo. Of the patients with a non-β0/β0 genotype, 11/14 (78.6%, 95% CI 49.2%-95.3%) achieved TI by Month 24 (Table 5). Among these 11 patients, the median (min, max) weighted average Hb during TI was 10.51 (9.3, 13.3) g/dL (Table 5).
All patients who have achieved TI at any time have maintained TI at Month 36 with a min, max duration of TI of 28.3+, 57.6+ months (N=11). The median (min, max) time to last RBC transfusion was 0.46 (0.2, 5.8) months following Zynteglo infusion.
In the 3 patients who did not achieve TI, reductions of 100%, 86.9% and 26.8% in transfusion volume requirements and of 100%, 85.3% and 20.7% in transfusion frequency were observed between Month 6 through Month 24 visit when compared to their pre-study levels of RBC transfusions. Reductions in volume and frequency were maintained at last follow-up in LTF-303.
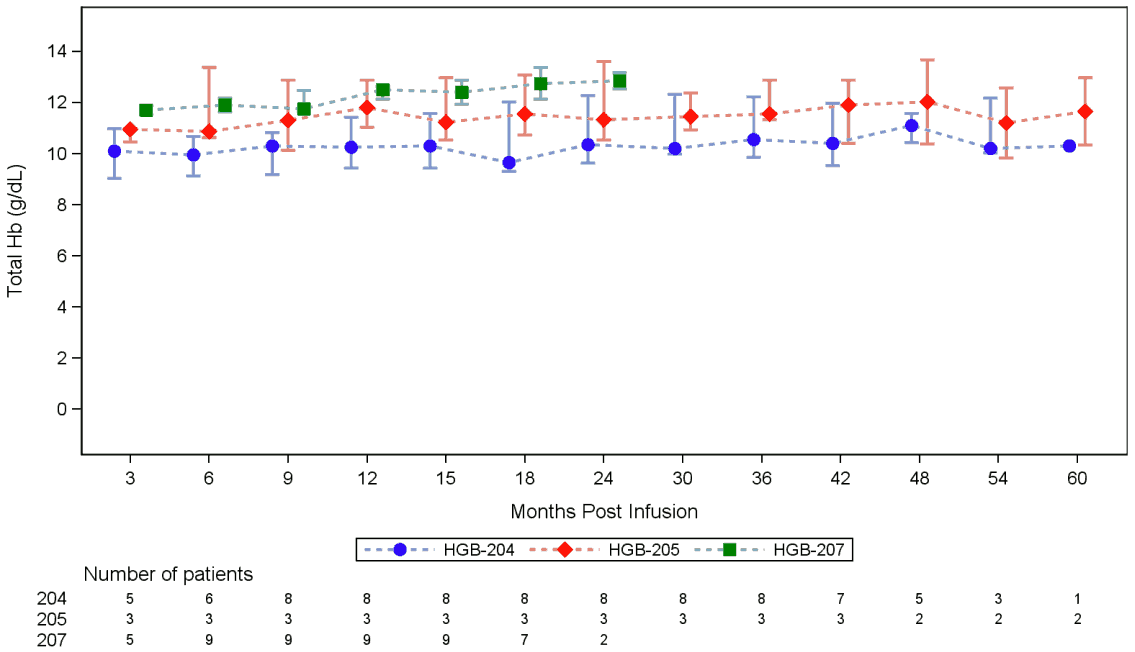
The median (min, max) total Hb at Month 6 for patients who had not received a transfusion for the prior 60 days was 10.60 (7.6, 13.4) g/dL (N=11). Total Hb remained stable at Month 24 with a median (min, max) of 10.60 (8.8, 13.7) g/dL (N=12) and at Month 36 with a median (min, max) of 10.60 (7.8, 13.5) g/dL (N=13).
After Zynteglo infusion, patient iron chelation was managed at physician discretion. Of the 14 non-β 0/β 0 patients treated in HGB-204 and HGB-205 that completed Month 6, 9 patients (64.3%) reported ongoing chelation use at last follow-up. The remaining 5 patients (35.7%) had stopped iron chelation, of whom 4 patients (28.6%) have stopped chelation for at least 6 months with median (min, max) time from stopping chelation to last follow-up of 26.40 (11.5, 42.2) months for these 4 patients. Additionally, of the 14 treated patients, 3 patients in HGB-205 (21.4%) received phlebotomy to remove iron. For the 11 patients that achieved TI, 4 patients (36.4%) stopped chelation for at least 6 months and 3 patients (27.3%) received phlebotomy.
At 48 months after infusion of Zynteglo for patients who achieved TI, the median reduction (min, max) in serum ferritin levels from baseline was 70.00% (39.2, 84.8) (N=5, HGB-204; N=2, HGB-205). The median reduction in liver iron content from baseline was 62.50%, ranging from an 83.3% reduction to a 269.2% increase (N=5, HGB-204; N=2, HGB-205).
Studies HGB-207 and HGB-212
HGB-207 and HGB-212 are ongoing Phase 3 open-label, single-arm 24-month studies that are planned to include approximately 39 adults, adolescents, and children with TDT (N=23, HGB-207; N=16, HGB-212), of whom 29 have a non-β0/β0 genotype (N=23, HGB-207; N=6, HGB-212) and 10 have a β0 /β0 genotype in HGB-212. These studies are conducted with improved transduction compared to Phase ½ studies, resulting in increased average number of functional copies of the transgene (βA-T87Qglobin) integrated in the autologous CD34+ cells. Eighteen adults and adolescents with TDT with a nonβ0/β0 genotype have been treated with Zynteglo in Phase 3 studies (N=15, HGB-207; N=3, HGB-212) and their median (min, max) duration of follow-up was 15.92 (5.6, 26.3) months. All patients remain alive at last follow-up.
The primary endpoint was transfusion independence (TI) by Month 24, defined as a weighted average Hb ≥9 g/dL without any RBC transfusions for a continuous period of ≥12 months at any time during the study after infusion of Zynteglo. Ten patients are evaluable for assessment of TI. Of these, 9/10 (90.0%, 95% CI 55.5-99.7%) achieved TI at last follow-up. Among these 9 patients, the median (min, max) weighted average Hb during TI was 12.22 (11.4, 12.8) g/dL (Table 5).
All patients who have achieved TI have maintained TI with a min, max duration of TI of 12.1+, 21.3+ months (N=9). The median (min, max) time to last RBC transfusion was 1.08 (0.5, 2.2) months following Zynteglo infusion.
For the only patient who did not achieve TI, a reduction of 51.5% in transfusion volume requirements and a reduction of 43.4% in transfusion frequency were observed from Month 12 to Month 24 when compared to their pre-study levels of RBC transfusions.
The median (min, max) total Hb at Month 6 for patients who had not received a transfusion for the prior 60 days was 11.85 (8.4, 13.3) g/dL (N=18). Total Hb remained stable at Month 24 with a median (min, max) of 12.85 (12.5, 13.2) g/dL (N=2).
After Zynteglo infusion, patient iron chelation was managed at physician discretion. Of the 18 non-β0/β0 patients treated in HGB-207 and HGB-212 that completed Month 6, 5 patients (27.8%) reported ongoing chelation use at last follow-up. The remaining 13 patients (72.2%) had stopped iron chelation, of whom 9 patients (50.0%) have stopped chelation for at least 6 months with median (min, max) time from stopping chelation to last follow-up of 16.89 (6.9, 25.4) months for these 9 patients. Additionally, of the 18 treated patients, 5 patients in HGB-207 (27.8%) received phlebotomy to remove iron. For the 9 patients that achieved TI, 6 patients (66.7%) stopped chelation for at least 6 months and 2 patients (22.2%) received phlebotomy.
Exploratory analyses were performed to evaluate resolution of dyserythropoiesis, the fundamental pathophysiological characteristic of TDT, in the bone marrow. Bone marrow biopsies taken before treatment were consistent with a diagnosis of TDT, including a low myeloid/erythroid ratio (N=15, HGB-207; N=3, HGB-212), reflective of erythroid hyperplasia. For 9 patients who achieved TI and had a Month 12 bone marrow assessment, myeloid/erythroid ratios increased from a median (min, max) of 0.2 (0.1 to 0.7) at baseline to a median (min, max) of 0.83 (0.6 to 1.9) at Month 12 after Zynteglo infusion, suggesting that Zynteglo improves erythropoiesis in patients with TDT.
Overall results
Figure 1 Median total haemoglobin over time in non-β0/β0 TDT patients treated with Zynteglo who have achieved transfusion independence (Studies HGB-204, HGB-205, HGB-207, LTF-303):
Bars represent interquartile ranges.
Total Hb represents those without any acute or chronic RBC transfusions within 60 days prior to the measurement date.
Table 5 Efficacy outcomes for non-β0/β0 TDT patients treated with Zynteglo (Studies HGB-204, HGB-205, HGB-207, HGB-212, LTF-303):
| HbAT87Q at 6 months (g/dL) n median (min, max) | HbAT87Q at 24 months (g/dL) n median (min, max) | Hb at 6 months* (g/dL) n median (min, max) | Hb at 24 months* (g/dL) n median (min, max) | TI** n/N^ () [95 CI] | WA Hb during TI (g/dL) n median (min, max) | Duration of TI (months) n median (min, max) |
|---|---|---|---|---|---|---|
| HGB-205 | ||||||
| 4 7.543 (4.94, 9.59) | 4 8.147 (6.72, 10.13) | 4 10.73 (7.6, 13.4) | 4 10.91 (8.8, 13.6) | 3/4 (75.0%) [19.4, 99.4] | 3 11.35 (10.5, 13.0) | 3 NR (38.2+, 57.6+) |
| HGB-204 | ||||||
| 10 4.153 (1.03, 8.52) | 10 5.418 (1.10, 9.60) | 7 9.20 (7.7, 13.3) | 8 10.35 (9.1, 13.7) | 8/10 (80.0%) [44.4, 97.5] | 8 10.27 (9.3, 13.3) | 8 NR (28.3+,51.3+) |
| HGB-207 | ||||||
| 13 9.324 (3.35, 10.60) | 3 8.766 (0.89, 11.40) | 15 11.80 (8.4, 13.3) | 2 12.85 (12.5, 13.2) | 9/10 (90.0%) [55.5, 99.7] | 9 12.22 (11.4, 12.8) | 9 NR (12.1+, 21.3+) |
| HGB-212 | ||||||
| 3 10.094 (5.06, 10.33) | NA*** | 3 12.10 (8.5, 12.2) | NA*** | NA*** | NA*** | NA*** |
* Patients who have not received transfusions in the prior 60 days.
** Transfusion independence (TI): a weighted average Hb ≥9 g/dL without any RBC transfusions for a continuous period of ≥12 months at any time during the study after medicinal product infusion.
*** No patients are currently evaluable for these endpoints.
N^ represents the total number of patients evaluable for TI, defined as patients who have completed their parent study (i.e., 24 months of follow-up), or achieved TI, or will not achieve TI in their parent study.
NR = Not reached. NA = Not applicable. Hb = Total Hb. WA Hb = Weighted average Hb.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Zynteglo in one or more subsets of the paediatric population in β-thalassaemia (see section 4.2 for information on paediatric use).
This medicinal product has been authorised under a so-called ‘conditional approval’ scheme. This means that further evidence on this medicinal product is awaited. The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
Zynteglo is an autologous gene therapy medicinal product consisting of autologous cells that have been genetically modified ex vivo. The nature of Zynteglo is such that conventional studies on pharmacokinetics, absorption, distribution, metabolism, and elimination are not applicable.
5.3. Preclinical safety data
Conventional mutagenicity, carcinogenicity and reproductive and developmental toxicity studies have not been conducted.
The pharmacology, toxicology and genotoxicity of the BB305 LVV used for transduction in the manufacture of Zynteglo were evaluated in vitro and in vivo. An in vitro immortalisation (IVIM) assay conducted with BB305 LVV-transduced mouse bone marrow cells (BMCs) showed minimal mutagenic potential (Fitness Score ≈ 0.1 × 10-4). Insertion site analysis (ISA) of pre-transplantation transduced mouse BMCs and human CD34+ HSCs showed no enrichment for insertion in or near cancer-related genes. A pharmacology, biodistribution, toxicity and genotoxicity study was conducted in a mouse model of β-thalassaemia. In this study, there was no evidence of toxicity, genotoxicity or oncogenesis (tumorigenicity) related to BB305 LVV integration, and no toxicity related to production of βA-T87Q-globin. ISA of post-transplantation BMCs demonstrated no preferred integration in the proximity of or within genes associated clinically (for gamma retroviral vectors) with either clonal dominance or leukaemia, and no evidence of clonal dominance was observed. Additional studies with human CD34+ HSCs administered to immunodeficient, myeloablated mice demonstrated no toxicity, tumorigenicity or genotoxicity.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.