Alglucosidase alfa
Interactions
Alglucosidase alfa interacts in the following cases:
Ulcerative and necrotizing skin lesions, nephrotic syndrome
Severe cutaneous reactions, possibly immune mediated, have been reported with alglucosidase alfa, including ulcerative and necrotizing skin lesions. Nephrotic syndrome was observed in a few Pompe patients treated with alglucosidase alfa and who had high IgG antibody titres (≥102,400). In these patients renal biopsy showed immune complex deposition. Patients improved following treatment interruption. It is therefore recommended to perform periodic urinalysis among patients with high IgG antibody titres.
Patients should be monitored for signs and symptoms of systemic immune-mediated reactions involving skin and other organs while receiving alglucosidase alfa. If immune-mediated reactions occur, discontinuation of the administration of alglucosidase alfa should be considered and appropriate medical treatment initiated. The risks and benefits of re-administering alglucosidase alfa following an immune-mediated reaction should be considered. Some patients have been successfully rechallenged and continued to receive alglucosidase alfa under close clinical supervision.
Hypersensitivity, anaphylactic reactions
Hypersensitivity/Anaphylactic reactions
Serious and life-threatening anaphylactic reactions, including anaphylactic shock, have been reported in infantile- and late-onset patients during alglucosidase alfa infusions. Because of the potential for severe infusion associated reactions, appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available when alglucosidase alfa is administered. If severe hypersensitivity or anaphylactic reactions occur, immediate discontinuation of alglucosidase alfa infusion should be considered and appropriate medical treatment should be initiated. The current medical standards for emergency treatment of anaphylactic reactions are to be observed.
Infusion Associated Reactions
Approximately half of the patients treated with alglucosidase alfa in infantile-onset clinical studies and 28% of the patients treated with alglucosidase alfa in a late-onset clinical study developed infusion associated reactions (IARs). IARs are defined as any related adverse event occurring during the infusion or during the hours following infusion. Some reactions were severe. A tendency was observed in infantile patients treated with a higher dose (40 mg/kg) to experience more symptoms when developing IARs. Infantile onset patients who develop high IgG antibody titres appear to be at higher risk for developing more frequent IARs. Patients with an acute illness (e.g. pneumonia, sepsis) at the time of alglucosidase alfa infusion appear to be at greater risk for IARs. Careful consideration should be given to the patient's clinical status prior to administration of alglucosidase alfa. Patients should be closely monitored and all cases of IARs, delayed reactions and possible immunological reactions should be reported to the marketing authorisation holder.
Patients who have experienced IARs (and in particular anaphylactic reactions) should be treated with caution when re-administering alglucosidase alfa. Mild and transient effects may not require medical treatment or discontinuation of the infusion. Reduction of the infusion rate, temporary interruption of the infusion, or pre-treatment, generally with oral antihistamine and/or antipyretics and/or corticosteroids, has effectively managed most reactions. IARs may occur at any time during the infusion of alglucosidase alfa or generally up to 2 hours after, and are more likely with higher infusion rates.
Patients with advanced Pompe disease may have compromised cardiac and respiratory function, which may predispose them to a higher risk of severe complications from infusion associated reactions. Therefore, these patients should be monitored more closely during administration of alglucosidase alfa.
Pregnancy
There are no data from the use of alglucosidase alfa in pregnant women. Studies in animals have shown reproductive toxicity. The potential risk for humans is unknown. Alglucosidase alfa should not be used during pregnancy unless clearly necessary.
Nursing mothers
Alglucosidase alfa may be excreted in breast milk. Because there are no data available on effects in neonates exposed to alglucosidase alfa via breast milk, it is recommended to stop breast-feeding when alglucosidase alfa is used.
Carcinogenesis, mutagenesis and fertility
Fertility
There are no clinical data on the effects of alglucosidase alfa on fertility. Preclinical data did not reveal any significant adverse findings.
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Because dizziness has been reported as an infusion associated reaction, this may affect the ability to drive and use machines on the day of the infusion.
Adverse reactions
Summary of the safety profile
Infantile-onset Pompe disease
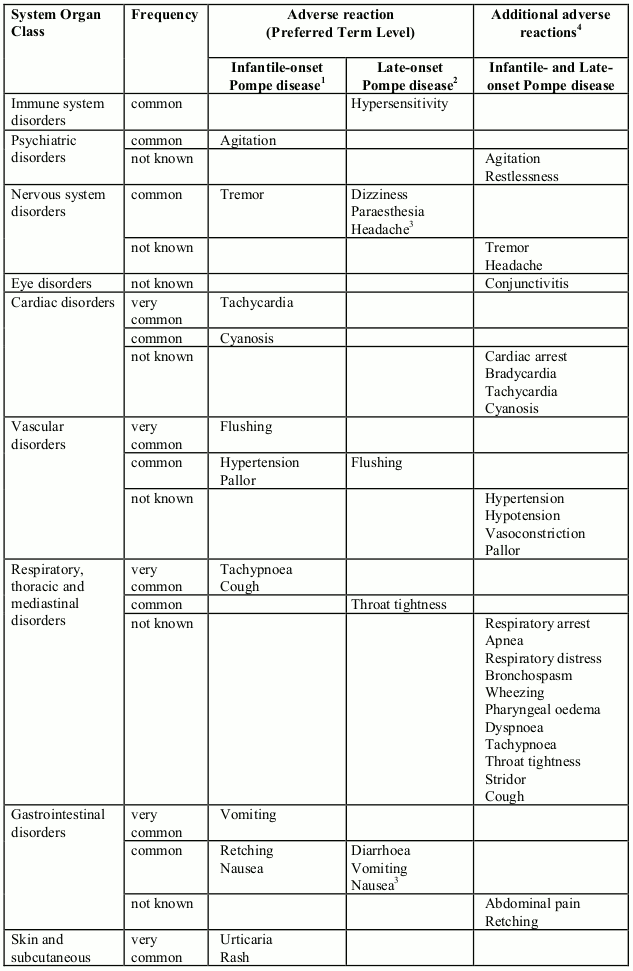
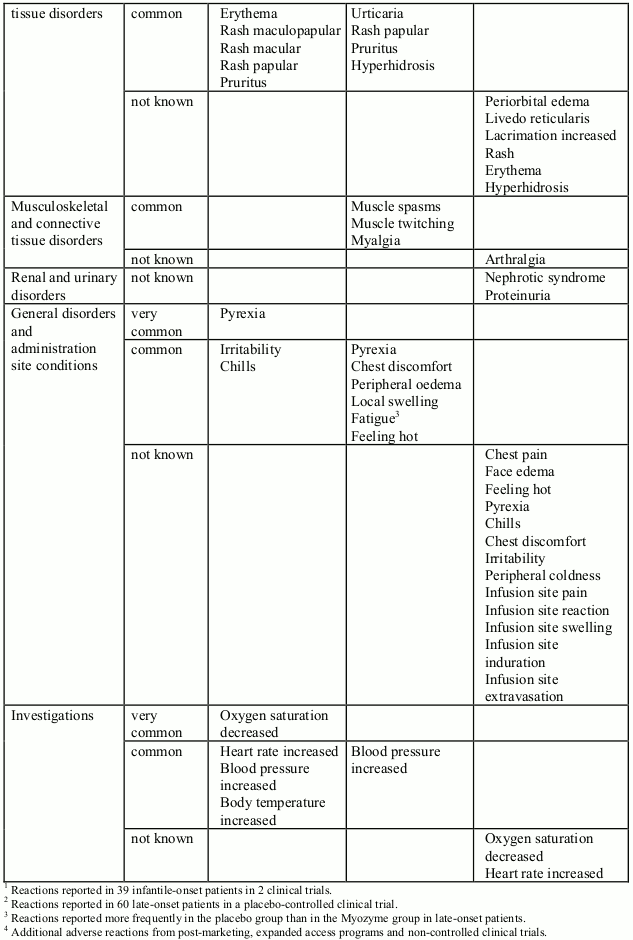
In clinical trials, 39 infantile-onset patients were treated with alglucosidase alfa for more than three years (168 weeks with a median of 121 weeks). Adverse reactions reported in at least 2 patients are listed in Table 1 by System Organ Class. Adverse reactions were mostly mild to moderate in intensity and almost all occurred during the infusion or during the 2 hours following the infusion (infusion associated reactions, IARs). Serious infusion reactions including urticaria, rales, tachycardia, decreased oxygen saturation, bronchospasm, tachypnea, periorbital edema and hypertension have been reported.
Late-onset Pompe disease
In a placebo-controlled study lasting 78 weeks, 90 patients with late-onset Pompe disease, aged 10 to 70 years, were treated with alglucosidase alfa or placebo randomized in a 2:1 ratio. Overall, the numbers of patients experiencing adverse reactions and serious adverse reactions were comparable between the two groups. The most common adverse reactions observed were IARs. Slightly more patients in the alglucosidase alfa group than in the placebo group experienced IARs (28% versus 23%). The majority of these reactions were non-serious, mild to moderate in intensity and resolved spontaneously. Adverse reactions reported in at least 2 patients are listed in Table 1. Serious adverse reactions reported in 4 patients treated with alglucosidase alfa were: angioedema, chest discomfort, throat tightness, non-cardiac chest pain and supraventricular tachycardia. Reactions in 2 of these patients were IgE-mediated hypersensitivity reactions.
Tabulated list of adverse reactions
Table 1: Adverse reactions (reported in at least 2 patients) and adverse reactions reported in postmarketing setting, expanded access programs and non-controlled clinical trials, per System Organ Class, presented by frequency categories: very common (≥1/10), common (≥1/100 to<1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000) and not known (cannot be estimated from the available data). Due to the small patient population, an adverse reaction reported in 2 patients is classified as common. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Description of selected adverse reactions
A small number of patients (<1%) in clinical trials and in the commercial setting developed anaphylactic shock and/or cardiac arrest during alglucosidase alfa infusion that required life-support measures. Reactions generally occurred shortly after initiation of the infusion. Patients presented with a constellation of signs and symptoms, primarily respiratory, cardiovascular, edematous and/or cutaneous in nature.
Recurrent reactions consisting of flu-like illness or a combination of events such as fever, chills, myalgia, arthralgia, pain, or fatigue occurring post-infusion and lasting usually for a few days, have been observed in some patients treated with alglucosidase alfa. The majority of patients were successfully re-challenged with alglucosidase alfa using lower doses and/or pretreatment with antiinflammatory drugs and/or corticosteroids and have continued to receive treatment under close clinical supervision.
Patients with moderate to severe or recurrent IARs have been evaluated for alglucosidase alfa specific IgE antibodies; some patients tested positive including some who experienced an anaphylactic reaction.
Nephrotic syndrome as well as severe cutaneous reactions, possibly immune mediated, have been reported with alglucosidase alfa including ulcerative and necrotizing skin lesions.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.