Atidarsagene autotemcel
Mechanism of action
Atidarsagene autotemcel is an ex vivo genetically modified autologous CD34+ hematopoietic stem and progenitor cell (HSPC) gene therapy. Autologous CD34+ HSPCs are collected from patient bone marrow (BM) harvest or from mobilised peripheral blood (mPB) and transduced with a lentiviral vector (ARSA LVV), which inserts one or more copies of the human ARSA complementary deoxyribonucleic acid (cDNA) into the cell’s genome, so that genetically modified cells become capable of expressing the functional ARSA enzyme. When administered to the patient following the administration of a myeloablative conditioning regimen, the genetically modified cells engraft and are able to repopulate the haematopoietic compartment. A subpopulation of the infused HSPCs and/or their myeloid progeny is able to migrate across the blood brain barrier to the brain and engraft as central nervous system (CNS) resident microglia and perivascular CNS macrophages as well as endoneural macrophages in the peripheral nervous system (PNS). These genetically modified cells can produce and secrete the functional ARSA enzyme, which can be taken up by surrounding cells, a process known as cross-correction, and used to break down, or prevent the build-up, of harmful sulfatides.
Following successful and stable engraftment in the patient, the effects of the product are expected to be persistent.
Pharmacodynamic properties
Durable and stable peripheral engraftment of genetically modified cells was observed from 1-month post atidarsagene autotemcel administration in all evaluable patients. A persistent vector copy number (VCN) was also observed in CD34+ cells isolated from the bone marrow throughout the follow-up period. These biological findings demonstrate a sustained multilineage engraftment of gene-corrected cells, which is essential for supporting the long-term production of ARSA and resulting long-term clinical benefit.
At Year 1 post-treatment, the proportion of BM-derived colonies harbouring the LVV genome (LV+) in the overall treated population was 54.8 (range: 20.0% to 100%, [N=23]). The proportion of BM-derived colonies harbouring the LVV genome (% LV+) at Year 5 was 45.0% (range: 18.8% to 90.6% [n=6, 4 Late infantile (LI) and 2 Early Juvenile (EJ)]), indicative of stable engraftment over time in the treated population.
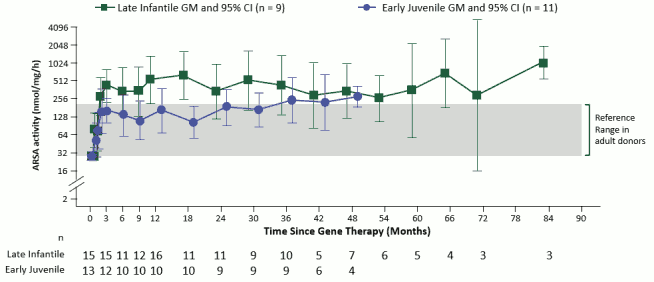
Reconstitution of ARSA activity in the hematopoietic system was observed in all MLD patients treated, with a progressive reconstitution of ARSA levels in Peripheral Blood Mononuclear Cells (PBMCs) which reached values within the normal reference range by 3 months post-treatment and remained stable within or above the normal range throughout the duration of the follow-up (see Figure 1).
Figure 1. ARSA activity in PBMCs over time (geometric mean and 95% CIs), by disease subtype (integrated efficacy set; N=29):
Note: Values < LLQ are imputed at LLQ. LLQ is 25.79 nmol/mg/h. GMs and 95% CIs are presented where there are at least 3 patients with non-missing data. ARSA: arylsulfatase A; CI: confidence interval; GM: geometric mean; LLQ: lower limit of quantification; PBMCs: peripheral blood mononuclear cells.
ARSA activity was also measured in cerebrospinal fluid (CSF) as a surrogate compartment of metabolic correction in the brain. The ARSA activity in CSF went from undetectable at Baseline to detectable in all evaluable patients by Month 6 post-treatment and reached reference range levels at Year 1 post-treatment. Thereafter, central reconstitution of ARSA enzymatic activity remained stable within the reference range.
Pharmacokinetic properties
Atidarsagene autotemcel is a gene therapy medicinal product consisting of autologous cells that have been genetically modified ex vivo. The nature of atidarsagene autotemcel is such that conventional studies on pharmacokinetics, absorption, metabolism, and elimination are not applicable. The biodistribution of atidarsagene autotemcel was nonetheless studied and distribution to haematopoietic tissues and disease target organs (including the brain) was demonstrated.
Preclinical safety data
Due to the nature of atidarsagene autotemcel, a standard toxicological assessment was not applicable and conventional mutagenicity, carcinogenicity and reproductive and developmental toxicity studies have not been conducted.
The pharmacology, toxicology and genotoxicity of atidarsagene autotemcel were evaluated in vitro and in vivo. Integration site analysis (ISA) of mouse Lin- bone marrow cells and human CD34+ cells transduced with ARSA LVV was conducted pre- and post-transplantation into mice and showed no enrichment for insertion in or near cancer-related genes, or clonal dominance. A prototype lentiviral vector related to ARSA LVV did not induce in vitro transformation and sustained growth of transduced wild type mouse Lin-bone marrow cells due to insertional transformation. Lin- bone marrow cells from Cdkn2a-/- mice, a strain prone to cancer triggered by gamma-retroviral insertional mutagenesis, transduced with the same prototype lentiviral vector did not show genotoxic potential when transplanted into wild type mice.
Toxicity and oncogenesis (tumorigenicity) studies were performed in the mouse model of MLD. No evidence of toxicity due to ARSA overexpression and no abnormal or malignant growth of transplanted cells or hematopoietic tumours related to the integration of ARSA LVV were observed. ARSA overexpression in human HSPCs and in ARSA Tg mice did not impair the activation of other sulfatases dependent on the sulfatase activator SUMF-1, did not affect the proliferation and differentiation capacities of transduced cells and did not induce toxicity or functional impairment in ARSA Tg mice.
Additional studies with human CD34+ cells transduced with ARSA LVV administered to immunodeficient, myeloablated mice demonstrated no toxicity, no vector mobilisation and bystander transduction of male gonads.
Molecular monitoring did not detect replication competent lentivirus (RCL).
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.