Cobicistat
Chemical formula: C₄₀H₅₃N₇O₅S₂ Molecular mass: 776.03 g/mol PubChem compound: 25151504
Pharmacodynamic properties
Cobicistat is a mechanism-based inhibitor of cytochrome P450 3A (CYP3A). Inhibition of CYP3A-mediated metabolism by cobicistat increases the systemic exposure of CYP3A substrates atazanavir and darunavir.
Pharmacokinetic properties
Absorption
In a trial where subjects were instructed to take coadministered TYBOST and darunavir with food, median cobicistat peak plasma concentrations were observed approximately 3.5 hours postdose. Steady-state cobicistat Cmax, AUCtau, and Ctau (mean ± SD) values were 0.99 ± 0.3 mcg/mL (n=60), 7.6 ± 3.7 mcg∙hr/mL (n=59), and 0.03 ± 0.1 mcg/mL (n=59), respectively.
Effect of Food on Oral Absorption
A food-effect trial was not conducted for TYBOST. In clinical trials, TYBOST was coadministered with other antiretroviral agents under fed conditions, in accordance with the prescribing information for these agents. It is recommended that TYBOST coadministered with atazanavir or darunavir be administered with food.
Distribution
Cobicistat is 97–98% bound to human plasma proteins and the mean blood-to-plasma ratio was approximately 0.5.
Metabolism
Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation.
Elimination
The terminal plasma half-life of cobicistat following administration of TYBOST is approximately 3 to 4 hours. With single dose administration of [ 14C] cobicistat after multiple dosing of cobicistat for 6 days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively.
Specific Populations
Race and Gender
No clinically relevant differences in the pharmacokinetics of cobicistat were observed based on race or gender.
Pediatric Patients
In pediatric subjects aged 12 to less than 18 years who received TYBOST 150 mg coadministered with atazanavir 300 mg (N=12), geometric mean atazanavir Cmax and AUCtau and cobicistat AUCtau values were approximately 20–30% higher than in adults and geometric mean atazanavir and cobicistat Ctau values were approximately 60% to 160% higher than in adults; the increases were not considered clinically significant. In pediatric subjects aged 12 to less than 18 years who received TYBOST 150 mg coadministered with darunavir 800 mg (N=7), geometric mean darunavir Cmax and AUCtau values were similar between adults and adolescents. Geometric mean darunavir AUCtau and Ctau values were 15% and 32% lower, with geometric mean ratios of 0.85 (90% CI: 0.64, 1.13) and 0.68 (90% CI: 0.30, 1.55) in adolescent subjects relative to adults, respectively. This difference was not considered clinically significant based on exposure-response relationships. Geometric mean cobicistat AUCtau, Cmax, and Ctau values were comparable in adolescents and adults (Table 9).
Table 9. Multiple-Dose PK Parameters of Cobicistat, Atazanavir, and Darunavir Following Administration of TYBOST with Atazanavir or Darunavir in HIV-1 Infected Pediatric Subjects Weighing at Least 35 kg*:
| Parameter Geometric Mean (CV%) | Cobicistat | Atazanavir | Darunavir | |
|---|---|---|---|---|
| Treatment Administered | TYBOST + Atazanavir | TYBOST + Darunavir | TYBOST + Atazanavir | TYBOST + Darunavir |
| Pediatric Subjects* | N=12 | N=7 | N=12 | N=7 |
| AUCtau (mcg∙hr/mL) | 12.11 (44.7) | 8.33 (34.9) | 49.48 (49.1) | 77.22 (29.5) |
| Cmax (mcg/mL) | 1.28 (31.7) | 1.10 (20.0) | 4.32 (49.9) | 7.32 (21.7) |
| Ctau (mcg/mL) | 0.09 (156.2) | 0.02 (123.9)† | 0.91 (96.4) | 0.68 (91.6) |
| Adults‡,§ | N=30‡ | N=21§ | N=30‡ | N=21§ |
| AUCtau (mcg∙hr/mL) | 9.65 (41.8) | 7.69 (43.9) | 39.96 (52.1) | 90.56 (45.3) |
| Cmax (mcg/mL) | 1.28 (35.6) | 1.04 (35.3) | 3.54 (45.8) | 8.34 (33.3) |
| Ctau (mcg/mL) | 0.04 (112.7) | 0.02 (135.1)¶ | 0.58 (84.7) | 1.00 (108.0) |
CV=Coefficient of Variation
* From Intensive PK analysis of Trial 128.
† N=5; Data from two subjects who had undetectable TYBOST Ctau concentrations were excluded from summary statistics.
‡ From pooled Intensive PK analysis of trials with TYBOST + atazanavir.
§ From Intensive PK analysis of Trial GS-US-299-0102 with TYBOST + darunavir.
¶ N=18.
Patients with Renal Impairment
No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with severe renal impairment (estimated creatinine clearance below 30 mL/min) and healthy subjects.
Patients with Hepatic Impairment
No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of cobicistat has not been studied.
Pregnancy and Postpartum
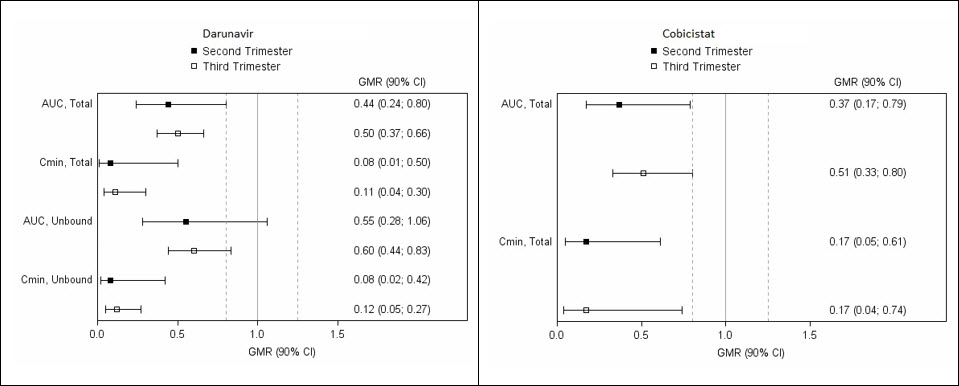
The exposure to total and unbound darunavir boosted with cobicistat after intake of darunavir/cobicistat as part of an antiretroviral regimen was substantially lower during the second and third trimesters of pregnancy compared with 6–12 weeks postpartum (see Table 1 and Figure 1).
Table 1. Pharmacokinetic Results of Total Darunavir after Administration of Darunavir/Cobicistat Once Daily as Part of an Antiretroviral Regimen, During the 2nd Trimester of Pregnancy, the 3 rd Trimester of Pregnancy, and Postpartum:
| Pharmacokinetics of total darunavir (mean ± SD) | 2nd Trimester of pregnancy N=7 | 3rd Trimester of pregnancy N=6 | Postpartum (6–12 weeks) N=6 |
|---|---|---|---|
| Cmax, ng/mL | 4,340 ± 1,616 | 4,910 ± 970 | 7,918 ± 2,199 |
| AUC24h, ng.h/mL | 47,293 ± 19,058 | 47,991 ± 9,879 | 99,613 ± 34,862 |
| Cmin, ng/mL | 168 ± 149 | 184 ± 99 | 1,538 ± 1,344 |
Figure 1. Pharmacokinetic Results (Within-Subject Comparison) of Total and Unbound Darunavir and Total Cobicistat After Administration of Darunavir/Cobicistat at 800/150 mg Once Daily as Part of an Antiretroviral Regimen, During the 2nd and 3rd Trimester of Pregnancy Compared to Postpartum:
Legend: 90% CI: 90% confidence interval; GMR: geometric mean ratio (i.e. second or third trimester/postpartum). Solid vertical line: ratio of 1.0; dotted vertical lines: reference lines of 0.8 and 1.25.
Assessment of Drug Interactions
Drug interaction trials were conducted with TYBOST (as a single entity) and desipramine, digoxin, and efavirenz. Drug interaction trials of TYBOST coadministered with atazanavir or darunavir included atorvastatin, drospirenone/ethinyl estradiol, and rosuvastatin. Drug interaction trials of TYBOST coadministered with elvitegravir included rosuvastatin and rifabutin.
The effects of cobicistat on the exposure of coadministered drugs are shown in Table 2.
Table 2. Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drugs in the Presence of Cobicistat*:
Note: The information listed below is not a comprehensive list of all the available drug interaction data for concomitant medications with cobicistat containing regimens. Please refer to the U.S. prescribing information for antiretroviral medications administered in combination with cobicistat for additional drug interaction information.
| Coadministered Drug | Dose of Coadministered Drug (mg) | TYBOST Dose (mg) | N | Mean Ratio of Coadministered Drug Pharmacokinetic Parameters (90% CI); No effect = 1.00 | |
|---|---|---|---|---|---|
| Cmax | AUC | ||||
| Atorvastatin | 10 single dose | 150 once daily | 16 | 18.85† (13.53, 26.27) | 9.22† (7.58, 11.22) |
| 4.19‡ (3.67, 4.78) | 3.90‡ (3.52, 4.32) | ||||
| Desipramine | 50 single dose | 150 once daily | 8 | 1.24 (1.08, 1.44) | 1.65 (1.36, 2.02) |
| Digoxin | 0.5 single dose | 150 once daily | 22 | 1.41 (1.29, 1.55) | 1.08 (1.00, 1.17) |
| Drospirenone/ ethinyl estradiol | 3 drospirenone single dose | 150 once daily | 14 | 1.12† (1.05, 1.19) | 2.30† (2.00, 2.64) |
| 0.02 ethinyl estradiol single dose | 0.82† (0.76, 0.89) | 0.78† (0.73, 0.85) | |||
| 3 drospirenone single dose | 150 once daily | 15 | 1.15‡ (1.05, 1.26) | 1.58‡ (1.47, 1.71) | |
| 0.02 ethinyl estradiol single dose | 0.86‡ (0.77, 0.95) | 0.70‡ (0.63, 0.77) | |||
| Efavirenz | 600 single dose | 150 once daily | 17 | 0.87 (0.80, 0.94) | 0.93 (0.89, 0.97) |
| Rosuvastatin | 10 single dose | 150 once daily | 16 | 10.58† (8.72, 12.83) | 3.42† (2.87, 4.07) |
| 3.77‡ (3.29, 4.32) | 1.93‡ (1.70, 2.20) | ||||
* All interaction studies conducted in healthy subjects.
† Study conducted in the presence of 300 mg atazanavir.
‡ Study conducted in the presence of 800 mg darunavir.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.