Docetaxel
Chemical formula: C₄₃H₅₃NO₁₄ Molecular mass: 807.879 g/mol PubChem compound: 148124
Interactions
Docetaxel interacts in the following cases:
P450-3A inhibitors, inducers, substrates
In vitro studies have shown that the metabolism of docetaxel may be modified by the concomitant administration of compounds which induce, inhibit or are metabolised by (and thus may inhibit the enzyme competitively) cytochrome P450-3A such as ciclosporine, ketoconazole and erythromycin. As a result, caution should be exercised when treating patients with these medicinal products as concomitant therapy since there is a potential for a significant interaction.
Strong CYP3A4 inhibitors
In case of combination with CYP3A4 inhibitors, the occurrence of docetaxel adverse reactions may increase, as a result of reduced metabolism. If the concomitant use of a strong CYP3A4 inhibitor (e.g., ketoconazole, itraconazole, clarithromycin, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole) cannot be avoided, a close clinical surveillance is warranted and a dose adjustment of docetaxel may be suitable during the treatment with the strong CYP3A4 inhibitor. In a pharmacokinetic study with 7 patients, the co-administration of docetaxel with the strong CYP3A4 inhibitor ketoconazole leads to a significant decrease in docetaxel clearance by 49%.
Liver impairment
In patients treated with docetaxel at 100 mg/m² as single agent who have serum transaminase levels (ALT and/or AST) greater than 1.5 times the ULN concurrent with serum alkaline phosphatase levels greater than 2.5 times the ULN, there is a higher risk of developing severe adverse reactions such as toxic deaths including sepsis and gastrointestinal haemorrhage which can be fatal, febrile neutropenia, infections, thrombocytopenia, stomatitis and asthenia. Therefore, the recommended dose of docetaxel in those patients with elevated liver function test (LFTs) is 75 mg/m² and LFTs should be measured at baseline and before each cycle.
For patients with serum bilirubin levels >ULN and/or ALT and AST >3.5 times the ULN concurrent with serum alkaline phosphatase levels >6 times the ULN, no dose-reduction can be recommended and docetaxel should not be used unless strictly indicated.
In combination with cisplatin and 5-fluorouracil for the treatment of patients with gastric adenocarcinoma, the pivotal clinical study excluded patients with ALT and/or AST >1.5 x ULN associated with alkaline phosphatase >2.5 x ULN, and bilirubin >1 x ULN; for these patients, no dose-reductions can be recommended and docetaxel should not be used unless strictly indicated. No data are available in patients with hepatic impairment treated by docetaxel in combination in the other indications.
Fertility
In non clinical studies, docetaxel has genotoxic effects and may alter male fertility. Therefore, men being treated with docetaxel are advised not to father a child during and up to 6 months after treatment and to seek advice on conservation of sperm prior to treatment.
Carboplatin
Limited data from a single uncontrolled study were suggestive of an interaction between docetaxel and carboplatin. When combined to docetaxel, the clearance of carboplatin was about 50% higher than values previously reported for carboplatin monotherapy.
Cystoid macular oedema (CMO)
Cystoid macular oedema (CMO) has been reported in patients treated with docetaxel. Patients with impaired vision should undergo a prompt and complete ophthalmologic examination. In case CMO is diagnosed, docetaxel treatment should be discontinued and appropriate treatment initiated.
Severe peripheral neurotoxicity
The development of severe peripheral neurotoxicity requires a reduction of dose.
Hypersensitivity reactions
Patients should be observed closely for hypersensitivity reactions especially during the first and second infusions. Hypersensitivity reactions may occur within a few minutes following the initiation of the infusion of docetaxel, thus facilities for the treatment of hypotension and bronchospasm should be available. If hypersensitivity reactions occur, minor symptoms such as flushing or localised cutaneous reactions do not require interruption of therapy. However, severe reactions, such as severe hypotension, bronchospasm or generalised rash/erythema require immediate discontinuation of docetaxel and appropriate therapy. Patients who have developed severe hypersensitivity reactions should not be re-challenged with docetaxel. Patients who have previously experienced a hypersensitivity reaction to paclitaxel may be at risk to develop hypersensitivity reaction to docetaxel, including more severe hypersensitivity reaction. These patients should be closely monitored during initiation of docetaxel therapy.
Congestive heart failure (CHF)
Patients should be monitored for symptoms of congestive heart failure during therapy and during the follow up period. In patients treated with the TAC regimen for node positive breast cancer, the risk of CHF has been shown to be higher during the first year after treatment.
Localised skin erythema
Localised skin erythema of the extremities (palms of the hands and soles of the feet) with oedema followed by desquamation has been observed. Severe symptoms such as eruptions followed by desquamation which lead to interruption or discontinuation of docetaxel treatment were reported.
Acute respiratory distress syndrome, interstitial pneumonia, interstitial lung disease, pulmonary fibrosis, respiratory failure
Acute respiratory distress syndrome, interstitial pneumonia/pneumonitis, interstitial lung disease, pulmonary fibrosis and respiratory failure have been reported and may be associated with fatal outcome. Cases of radiation pneumonitis have been reported in patients receiving concomitant radiotherapy.
If new or worsening pulmonary symptoms develop, patients should be closely monitored, promptly investigated, and appropriately treated. Interruption of docetaxel therapy is recommended until diagnosis is available. Early use of supportive care measures may help improve the condition. The benefit of resuming docetaxel treatment must be carefully evaluated.
Enterocolitis, gastrointestinal toxicity
Caution is recommended for patients with neutropenia, particularly at risk for developing gastrointestinal complications. Although majority of cases occurred during the first or second cycle of docetaxel containing regimen, enterocolitis could develop at any time, and could lead to death as early as on the first day of onset. Patients should be closely monitored for early manifestations of serious gastrointestinal toxicity.
Pregnancy
There is no information on the use of docetaxel in pregnant women. Docetaxel has been shown to be both embryotoxic and foetotoxic in rabbits and rats, and to reduce fertility in rats. As with other cytotoxic medicinal products, docetaxel may cause foetal harm when administered to pregnant women. Therefore, docetaxel must not be used during pregnancy unless clearly indicated.
Women of childbearing age receiving docetaxel should be advised to avoid becoming pregnant, and to inform the treating physician immediately should this occur.
Nursing mothers
Docetaxel is a lipophilic substance but it is not known whether it is excreted in human milk. Consequently, because of the potential for adverse reactions in nursing infants, breast feeding must be discontinued for the duration of docetaxel therapy.
Carcinogenesis, mutagenesis and fertility
Contraception in males and females
An effective method of contraception should be used during treatment.
Fertility
In non clinical studies, docetaxel has genotoxic effects and may alter male fertility. Therefore, men being treated with docetaxel are advised not to father a child during and up to 6 months after treatment and to seek advice on conservation of sperm prior to treatment.
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. The amount of alcohol in this medicinal product and the side effects of the product may impair the ability to drive or use machines. Therefore, patients should be warned of the potential impact of the amount of alcohol and the side effects of this medicinal product on the ability to drive or use machines, and be advised not to drive or use machines if they experience these side effects during treatment.
Adverse reactions
Summary of the safety profile for all indications
The adverse reactions considered to be possibly or probably related to the administration of docetaxel have been obtained in:
- 1,312 and 121 patients who received 100 mg/m² and 75 mg/m² of docetaxel as a single agent respectively.
- 258 patients who received docetaxel in combination with doxorubicin.
- 406 patients who received docetaxel in combination with cisplatin.
- 92 patients treated with docetaxel in combination with trastuzumab.
- 255 patients who received docetaxel in combination with capecitabine.
- 332 patients who received docetaxel in combination with prednisone or prednisolone (clinically important treatment related adverse events are presented).
- 1,276 patients (744 and 532 in TAX 316 and GEICAM 9805 respectively) who received docetaxel in combination with doxorubicin and cyclophosphamide (clinically important treatment related adverse events are presented).
- 300 gastric adenocarcinoma patients (221 patients in the phase III part of the study and 79 patients in the phase II part) who received docetaxel in combination with cisplatin and 5-fluorouracil (clinically important treatment related adverse events are presented).
- 174 and 251 head and neck cancer patients who received docetaxel in combination with cisplatin and 5-fluorouracil (clinically important treatment related adverse events are presented).
These reactions were described using the NCI Common Toxicity Criteria (grade 3 = G3; grade 3-4 = G3/4; grade 4 = G4), the COSTART and the MedDRA terms. Frequencies are defined as: very common (>1/10), common (>1/100 to <1/10); uncommon (>1/1,000 to <1/100); rare (>1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data).
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
The most commonly reported adverse reactions of docetaxel alone are: neutropenia (which was reversible and not cumulative; the median day to nadir was 7 days and the median duration of severe neutropenia (<500 cells/mm³) was 7 days), anaemia, alopecia, nausea, vomiting, stomatitis, diarrhoea and asthenia. The severity of adverse events of docetaxel may be increased when docetaxel is given in combination with other chemotherapeutic agents.
For combination with trastuzumab, adverse events (all grades) reported in >10% are displayed. There was an increased incidence of SAEs (40% vs. 31%) and Grade 4 AEs (34% vs. 23%) in the trastuzumab combination arm compared to docetaxel monotherapy.
For combination with capecitabine, the most frequent treatment-related undesirable effects (>5%) reported in a phase III study in breast cancer patients failing anthracycline treatment are presented (see capecitabine summary of product characteristics).
The following adverse reactions are frequently observed with docetaxel:
Immune system disorders
Hypersensitivity reactions have generally occurred within a few minutes following the start of the infusion of docetaxel and were usually mild to moderate. The most frequently reported symptoms were flushing, rash with or without pruritus, chest tightness, back pain, dyspnoea and fever or chills. Severe reactions were characterised by hypotension and/or bronchospasm or generalized rash/erythema.
Nervous system disorders
The development of severe peripheral neurotoxicity requires a reduction of dose. Mild to moderate neuro-sensory signs are characterised by paraesthesia, dysesthesia or pain including burning. Neuro-motor events are mainly characterised by weakness.
Skin and subcutaneous tissue disorders
Reversible cutaneous reactions have been observed and were generally considered as mild to moderate. Reactions were characterised by a rash including localised eruptions mainly on the feet and hands (including severe hand and foot syndrome), but also on the arms, face or thorax, and frequently associated with pruritus. Eruptions generally occurred within one week after the docetaxel infusion. Less frequently, severe symptoms such as eruptions followed by desquamation which rarely lead to interruption or discontinuation of docetaxel treatment were reported. Severe nail disorders are characterised by hypo- or hyperpigmentation and sometimes pain and onycholysis.
General disorders and administration site conditions
Infusion site reactions were generally mild and consisted of hyper pigmentation, inflammation, redness or dryness of the skin, phlebitis or extravasation and swelling of the vein. Fluid retention includes events such as peripheral oedema and less frequently pleural effusion, pericardial effusion, ascites and weight gain. The peripheral oedema usually starts at the lower extremities and may become generalised with a weight gain of 3 kg or more. Fluid retention is cumulative in incidence and severity.
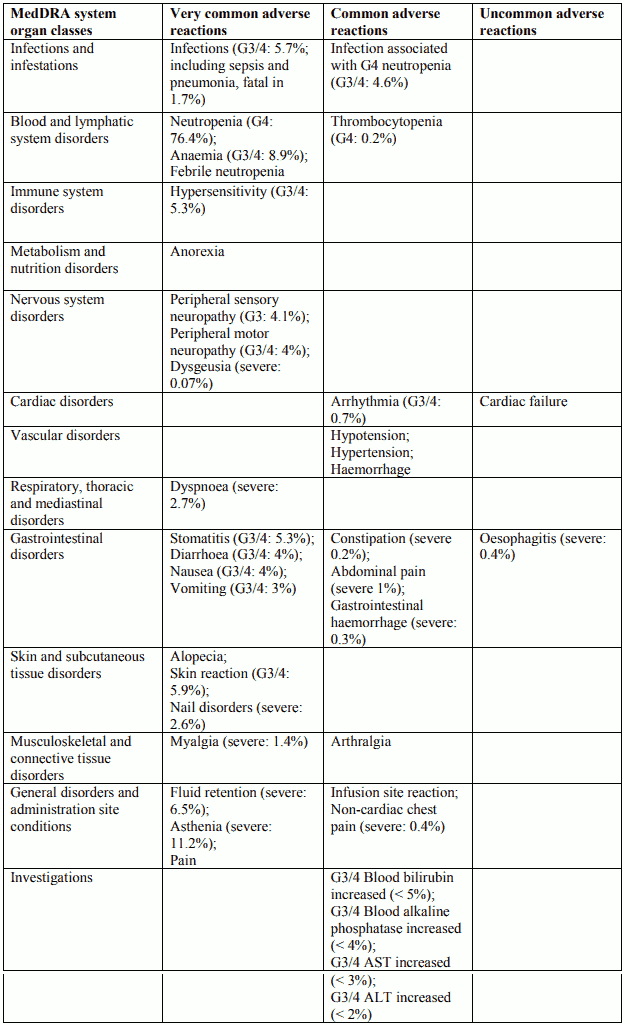
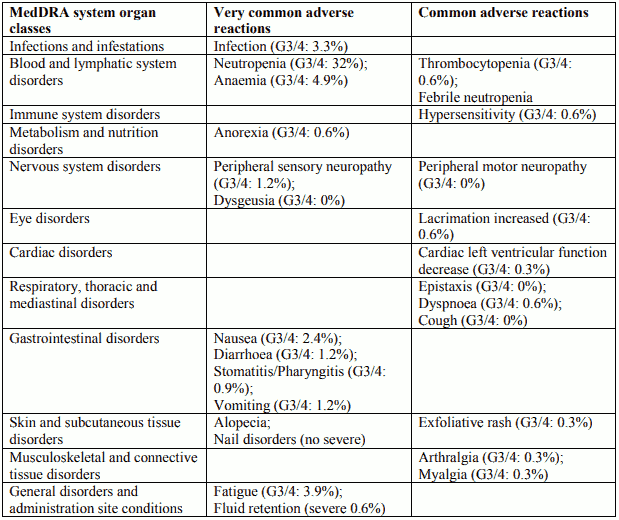
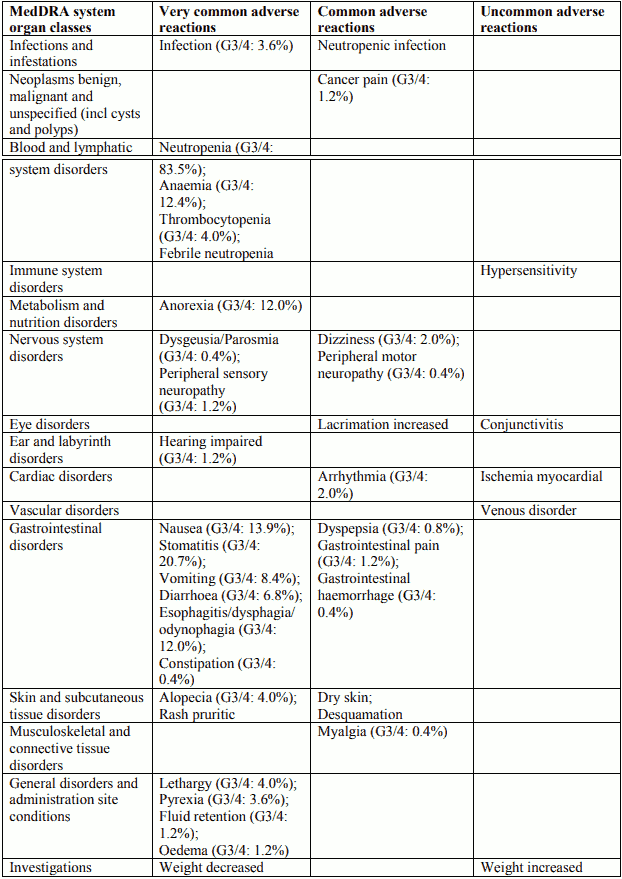
Tabulated list of adverse reactions in breast cancer for Docetaxel 100 mg/m² single agent
Description of selected adverse reactions in breast cancer for Docetaxel 100 mg/m² single agent
Blood and lymphatic system disorders
Rare: bleeding episodes associated with grade ¾ thrombocytopenia.
Nervous system disorders
Reversibility data are available among 35.3% of patients who developed neurotoxicity following docetaxel treatment at 100 mg/m² as single agent. The events were spontaneously reversible within 3 months.
Skin and subcutaneous tissue disorders
Very rare: one case of alopecia non-reversible at the end of the study. 73% of the cutaneous reactions were reversible within 21 days.
General disorders and administration site conditions
The median cumulative dose to treatment discontinuation was more than 1,000 mg/m² and the median time to fluid retention reversibility was 16.4 weeks (range 0 to 42 weeks). The onset of moderate and severe retention is delayed (median cumulative dose: 818.9 mg/m²) in patients with premedication compared with patients without premedication (median cumulative dose: 489.7 mg/m²); however, it has been reported in some patients during the early courses of therapy.
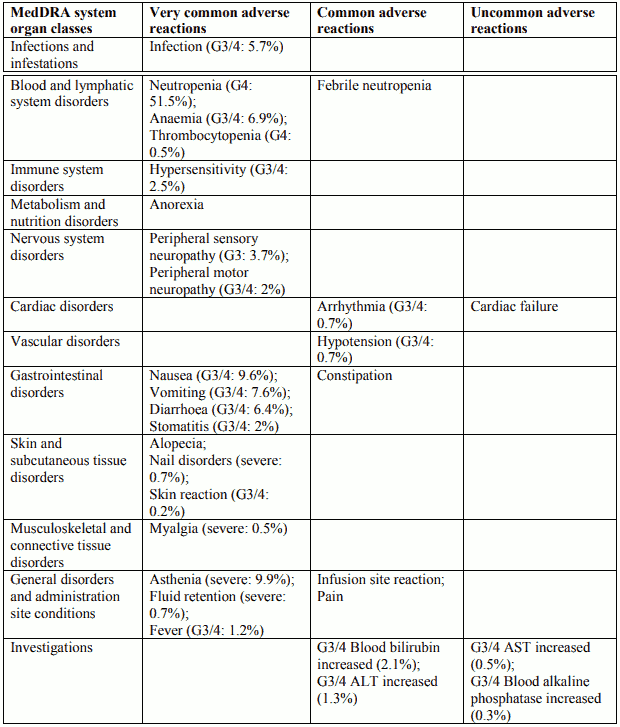
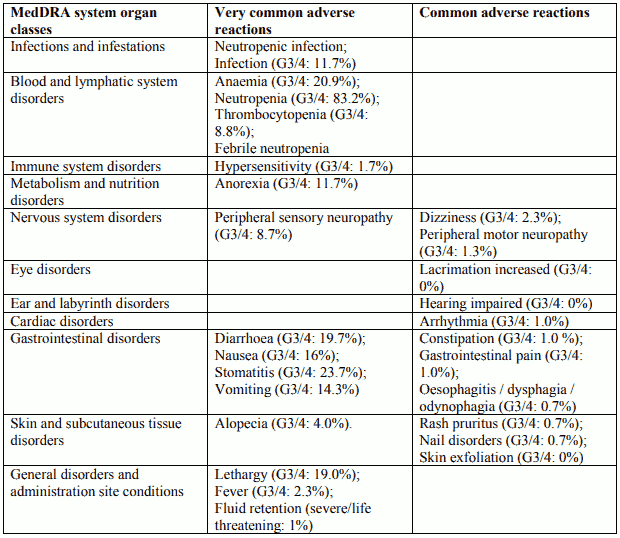
Tabulated list of adverse reactions in non-small cell lung cancer for Docetaxel 75 mg/m² single agent
Tabulated list of adverse reactions in breast cancer for Docetaxel 75 mg/m² in combination with doxorubicin
Tabulated list of adverse reactions in non-small cell lung cancer for Docetaxel 75 mg/m² in combination with cisplatin
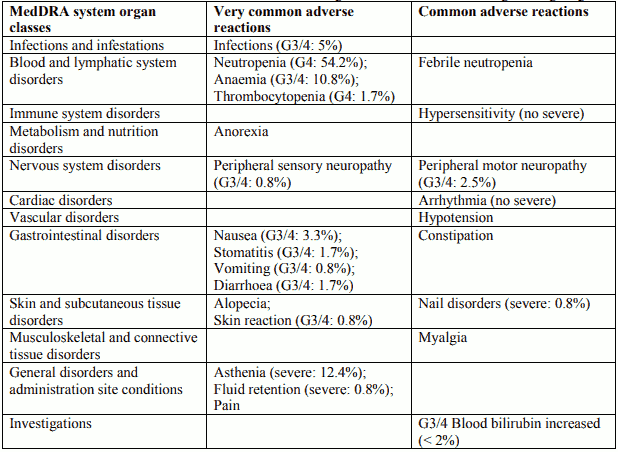
Tabulated list of adverse reactions in breast cancer for Docetaxel 100 mg/m² in combination with trastuzumab
Description of selected adverse reactions in breast cancer for Docetaxel 100 mg/m² in combination with trastuzumab
Cardiac disorders
Symptomatic cardiac failure was reported in 2.2% of the patients who received docetaxel plus trastuzumab compared to 0% of patients given docetaxel alone. In the docetaxel plus trastuzumab arm, 64% had received a prior anthracycline as adjuvant therapy compared with 55% in the docetaxel arm alone.
Blood and lymphatic system disorders
Very common: Haematological toxicity was increased in patients receiving trastuzumab and docetaxel, compared with docetaxel alone (32% grade ¾ neutropenia versus 22%, using NCI-CTC criteria). Note that this is likely to be an underestimate since docetaxel alone at a dose of 100 mg/m² is known to result in neutropenia in 97% of patients, 76% grade 4, based on nadir blood counts. The incidence of febrile neutropenia/neutropenic sepsis was also increased in patients treated with Herceptin plus docetaxel (23% versus 17% for patients treated with docetaxel alone).
Tabulated list of adverse reactions in breast cancer for Docetaxel 75 mg/m² in combination with capecitabine
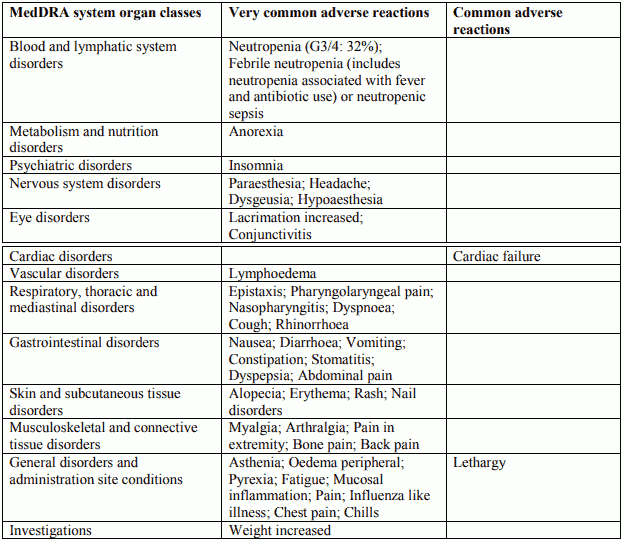
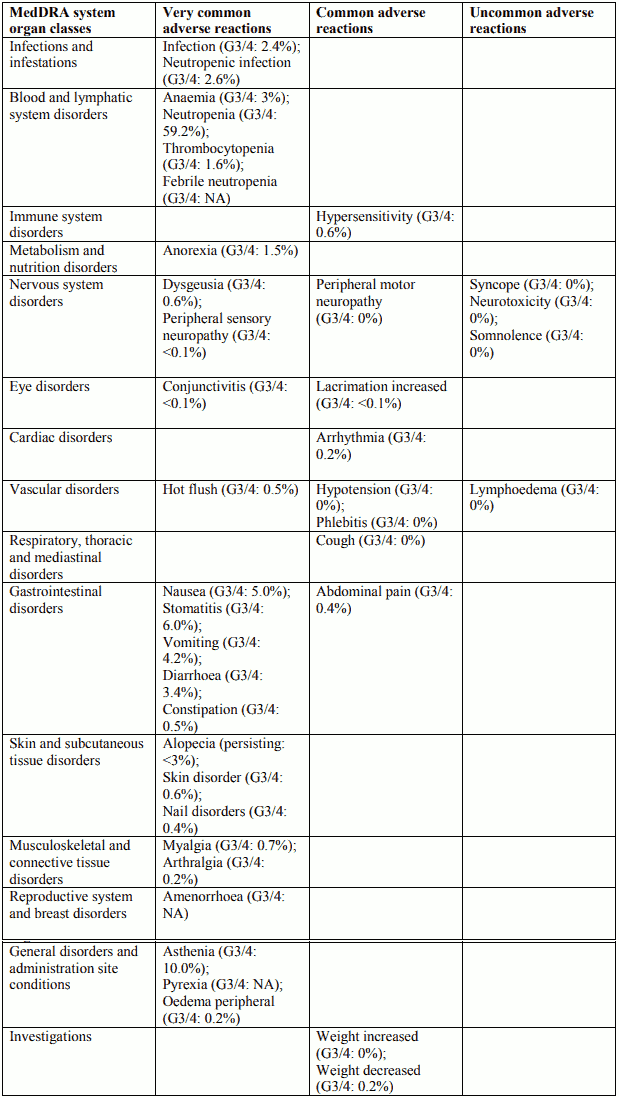
Tabulated list of adverse reactions in prostate cancer for Docetaxel 75 mg/m² in combination with prednisone or prednisolone
Tabulated list of adverse reactions for adjuvant therapy with Docetaxel 75 mg/m² in combination with doxorubicin and cyclophosphamide in patients with node-positive (TAX 316) and node-negative (GEICAM 9805) breast cancer – pooled data
Description of selected adverse reactions for adjuvant therapy with Docetaxel 75 mg/m² in combination with doxorubicin and cyclophosphamide in patients with node-positive (TAX 316) and node-negative (GEICAM 9805) breast cancer
Nervous system disorders
In study TAX316 peripheral sensory neuropathy started during the treatment period and persisted into the follow-up period in 84 patients (11.3%) in TAC arm and 15 patients (2%) in FAC arm. At the end of the follow-up period (median follow-up time of 8 years), peripheral sensory neuropathy was observed to be ongoing in 10 patients (1.3%) in TAC arm, and in 2 patients (0.3%) in FAC arm. In GEICAM 9805 study peripheral sensory neuropathy that started during the treatment period persisted into the follow-up period in 10 patients (1.9%) in TAC arm and 4 patients (0.8%) in FAC arm. At the end of the follow-up period (median follow-up time of 10 years and 5 months), peripheral sensory neuropathy was observed to be ongoing in 3 patients (0.6%) in TAC arm, and in 1 patient (0.2%) in FAC arm.
Cardiac disorders
In study TAX316, 26 patients (3.5%) in the TAC arm and 17 patients (2.3%) in the FAC arm experienced congestive heart failure. All except one patient in each arm were diagnosed with CHF more than 30 days after the treatment period. Two patients in the TAC arm and 4 patients in the FAC arm died because of cardiac failure.
In GEICAM 9805 study, 3 patients (0.6%) in TAC arm and 3 patients (0.6%) in FAC arm developed congestive heart failure during the follow-up period. At the end of the follow-up period (actual median follow-up time of 10 years and 5 months), no patients had CHF in TAC arm and 1 patient in TAC arm died because of dilated cardiomyopathy, and CHF was observed to be ongoing in 1 patient (0.2%) in FAC arm.
Skin and subcutaneous tissue disorders
In study TAX316 alopecia persisting into the follow-up period after the end of chemotherapy was reported in 687 of 744 TAC patients (92.3%) and 645 of 736 FAC patients (87.6%). At the end of the follow-up period (actual median follow-up time of 8 years), alopecia was observed to be ongoing in 29 TAC patients (3.9%) and 16 FAC patients (2.2%).
In GEICAM 9805 study alopecia that started during the treatment period and persisted into the follow- up period was observed to be ongoing in 49 patients (9.2%) in TAC arm and 35 patients (6.7%) in FAC arm. Alopecia related to study drug started or worsened during the follow-up period in 42 patients (7.9%) in TAC arm and 30 patients (5.8%) in FAC arm. At the end of the follow-up period (median follow-up time of 10 years and 5 months), alopecia was observed to be ongoing in 3 patients (0.6%) in TAC arm, and in 1 patient (0.2%) in FAC arm.
Reproductive system and breast disorders
In TAX316 amenorrhoea that started during the treatment period and persisted into the follow-up period after the end of chemotherapy was reported in 202 of 744 TAC patients (27.2%) and 125 of 736 FAC patients (17.0%). Amenorrhea was observed to be ongoing at the end of the follow-up period (median follow-up time of 8 years) in 121 of 744 TAC patients (16.3%) and 86 FAC patients (11.7%). In GEICAM 9805 study amenorrhoea that started during the treatment period and persisted into the follow-up period was observed to be ongoing in 18 patients (3.4%) in TAC arm and 5 patients (1.0%) in FAC arm. At the end of the follow-up period (median follow-up time of 10 years and 5 months), amenorrhoea was observed to be ongoing in 7 patients (1.3%) in TAC arm, and in 4 patients (0.8%) in FAC arm.
General disorders and administration site conditions
In study TAX316 peripheral oedema that started during the treatment period and persisted into the follow-up period after the end of chemotherapy was observed in 119 of 744 TAC patients (16.0%) and 23 of 736 FAC patients (3.1%). At the end of the follow-up period (actual median follow-up time of 8 years), peripheral oedema was ongoing in 19 TAC patients (2.6%) and 4 FAC patients (0.5%). In study TAX316 lymphoedema that started during the treatment period and persisted into the follow- up period after the end of chemotherapy was reported in 11 of 744 TAC patients (1.5%) and 1 of 736 FAC patients (0.1%). At the end of the follow-up period (actual median follow-up time of 8 years), lymphoedema was observed to be ongoing in 6 TAC patients (0.8%) and 1 FAC patient (0.1%). In study TAX316 asthenia that started during the treatment period and persisted into the follow-up period after the end of chemotherapy was reported in 236 of 744 TAC patients (31.7%) and 180 of 736 FAC patients (24.5%). At the end of the follow-up period (actual median follow-up time of 8 years), asthenia was observed to be ongoing in 29 TAC patients (3.9%) and 16 FAC patients (2.2%).
In study GEICAM 9805 peripheral oedema that started during the treatment period persisted into the follow-up period in 4 patients (0.8%) in TAC arm and in 2 patients (0.4%) in FAC arm. At the end of the follow-up period (median follow-up time of 10 years and 5 months), no patients (0%) in TAC arm had peripheral oedema and it was observed to be ongoing in 1 patient (0.2%) in FAC arm. Lymphoedema that started during the treatment period persisted into the follow-up period in 5 patients (0.9%) in TAC arm and 2 patients (0.4%) in FAC arm. At the end of the follow-up period, lymphoedema was observed to be ongoing in 4 patients (0.8%) in TAC arm, and in 1 patient (0.2%) in FAC arm.
Asthenia that started during the treatment period and persisted into the follow-up period was observed to be ongoing in 12 patients (2.3%) in TAC arm and 4 patients (0.8%) in FAC arm. At the end of the follow-up period, asthenia was observed to be ongoing in 2 patients (0.4%) in TAC arm, and in 2 patients (0.4%) in FAC arm.
Acute leukaemia / Myelodysplastic syndrome.
After 10 years of follow up in study TAX316, acute leukaemia was reported in 3 of 744 TAC patients (0.4%) and in 1 of 736 FAC patients (0.1%). One TAC patient (0.1%) and 1 FAC patient (0.1%) died due to AML during the follow-up period (median follow-up time of 8 years). Myelodysplastic syndrome was reported in 2 of 744 TAC patients (0.3%) and in 1 of 736 FAC patients (0.1%). After 10 years of follow-up in GEICAM 9805 study, acute leukaemia occurred in 1 of 532 (0.2%) patients in TAC arm. No cases were reported in patients in FAC arm. No patient was diagnosed with myelodysplastic syndrome in either treatment groups.
Neutropenic complications
Table below shows that the incidence of Grade 4 neutropenia, febrile neutropenia and neutropenic infection was decreased in patients who received primary G-CSF prophylaxis after it was made mandatory in the TAC arm – GEICAM study.
Neutropenic complications in patients receiving TAC with or without primary G-CSF prophylaxis (GEICAM 9805)
| Without primary G-CSF prophylaxis (n=111) n(%) | With primary G-CSF prophylaxis (n=421) n(%) | |
|---|---|---|
| Neutropenia (Grade 4) | 104 (93.7) | 135 (32.1) |
| Febrile neutropenia | 28 (25.2) | 23 (5.5) |
| Neutropenic infection | 14 (12.6) | 21 (5.0) |
| Neutropenic infection (Grade 3-4) | 2 (1.8) | 5 (1.2) |
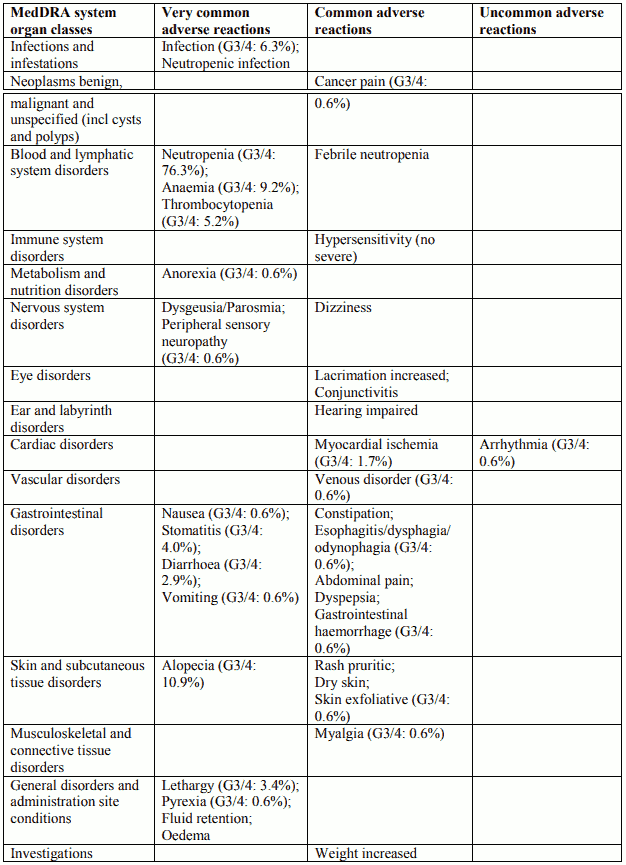
Tabulated list of adverse reactions in gastric adenocarcinoma cancer for Docetaxel 75 mg/m² in combination with cisplatin and 5-fluorouracil:
Description of selected adverse reactions in gastric adenocarcinoma cancer for Docetaxel 75 mg/m² in combination with cisplatin and 5-fluorouracil
Blood and lymphatic system disorders
Febrile neutropenia and neutropenic infection occurred in 17.2% and 13.5% of patients respectively, regardless of G-CSF use. G-CSF was used for secondary prophylaxis in 19.3% of patients (10.7% of the cycles). Febrile neutropenia and neutropenic infection occurred respectively in 12.1% and 3.4% of patients when patients received prophylactic G-CSF, in 15.6% and 12.9% of patients without prophylactic G-CSF.
Tabulated list of adverse reactions in head and neck cancer for Docetaxel 75 mg/m² in combination with cisplatin and 5-fluorouracil
Induction chemotherapy followed by radiotherapy (TAX 323)
Induction chemotherapy followed by chemoradiotherapy (TAX 324)
Post-marketing experience
Neoplasms benign, malignant and unspecified (incl cysts and polyps)
Second primary malignancies (frequency not known), including non-Hodgkin lymphoma have been reported in association with docetaxel when used in combination with other anticancer treatments known to be associated with second primary malignancies. Acute myeloid leukemia and myelodysplastic syndrome have been reported (frequency uncommon) in pivotal clinical studies in breast cancer with TAC regimen.
Blood and lymphatic system disorders
Bone marrow suppression and other haematologic adverse reactions have been reported. Disseminated intravascular coagulation (DIC), often in association with sepsis or multi-organ failure, has been reported.
Immune system disorders
Some cases of anaphylactic shock, sometimes fatal, have been reported.
Hypersensitivity reactions (frequency not known) have been reported with docetaxel in patients who previously experienced hypersensitivity reactions to paclitaxel.
Nervous system disorders
Rare cases of convulsion or transient loss of consciousness have been observed with docetaxel administration. These reactions sometimes appear during the infusion of the medicinal product.
Eye disorders
Very rare cases of transient visual disturbances (flashes, flashing lights, scotomata) typically occurring during infusion of the medicinal product and in association with hypersensitivity reactions have been reported. These were reversible upon discontinuation of the infusion. Cases of lacrimation with or without conjunctivitis, as cases of lacrimal duct obstruction resulting in excessive tearing have been rarely reported. Cases of cystoid macular oedema (CMO) have been reported in patients treated with docetaxel.
Ear and labyrinth disorders
Rare cases of ototoxicity, hearing impaired and/or hearing loss have been reported.
Cardiac disorders
Rare cases of myocardial infarction have been reported.
Ventricular arrhythmia including ventricular tachycardia (frequency not known), sometimes fatal, has been reported in patients treated with docetaxel in combination regimens including doxorubicin, 5- fluorouracil and/ or cyclophosphamide.
Vascular disorders
Venous thromboembolic events have rarely been reported.
Respiratory, thoracic and mediastinal disorders
Acute respiratory distress syndrome and cases of interstitial pneumonia/pneumonitis, interstitial lung disease, pulmonary fibrosis and respiratory failure sometimes fatal have rarely been reported. Rare cases of radiation pneumonitis have been reported in patients receiving concomitant radiotherapy.
Gastrointestinal disorders
Rare cases of enterocolitis, including colitis, ischemic colitis, and neutropenic enterocolitis, have been reported with a potential fatal outcome (frequency not known).
Rare occurrences of dehydration have been reported as a consequence of gastrointestinal events including enterocolitis and gastrointestinal perforation.
Rare cases of ileus and intestinal obstruction have been reported.
Hepatobiliary disorders
Very rare cases of hepatitis, sometimes fatal primarily in patients with pre-existing liver disorders, have been reported.
Skin and subcutaneous tissue disorders
Very rare cases of cutaneous lupus erythematosus and bullous eruptions such as erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, have been reported with docetaxel. In some cases concomitant factors may have contributed to the development of these effects. Scleroderma-like changes usually preceded by peripheral lymphoedema have been reported with docetaxel. Cases of permanent alopecia (frequency not known) have been reported.
Renal and urinary disorders
Renal insufficiency and renal failure have been reported. In about 20% of these cases there were no risk factors for acute renal failure such as concomitant nephrotoxic medicinal products and gastrointestinal disorders.
General disorders and administration site conditions
Radiation recall phenomena have rarely been reported.
Injection site recall reaction (recurrence of skin reaction at a site of previous extravasation following administration of docetaxel at a different site) has been observed at the site of previous extravasation (frequency not known).
Fluid retention has not been accompanied by acute episodes of oliguria or hypotension. Dehydration and pulmonary oedema have rarely been reported.
Metabolism and nutrition disorders
Cases of electrolyte imbalance have been reported. Cases of hyponatraemia have been reported, mostly associated with dehydration, vomiting and pneumonia. Hypokalaemia, hypomagnesaemia, and hypocalcaemia were observed, usually in association with gastrointestinal disorders and in particular with diarrhoea.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.