Efbemalenograstim alfa
Pharmacodynamic properties
Human granulocyte-colony stimulating factor (G-CSF) is a glycoprotein, which regulates the production and release of neutrophils from the bone marrow.
Efbemalenograstim alfa is a recombinant fusion protein containing G-CSF, a 16 amino-acid linker, and the Fc portion of human IgG2. In solution, efbemalenograstim alfa forms covalentlylinked dimers (disulfide bonds between Fc moieties) and has an immunoglobulin-like structure. Efbemalenograstim alfa is a sustained duration form of G-CSF due to decreased renal clearance. Efbemalenograstim alfa and other G-CSFs have identical modes of action, causing a marked increase in peripheral blood neutrophil counts within 24 hours, with minor increases in monocytes and/or lymphocytes.
Neutrophils produced in response to G-CSF show normal or enhanced function as demonstrated by tests of chemotactic and phagocytic function. As with other haematopoietic growth factors, G-CSF has shown in vitro stimulating properties on human endothelial cells. G-CSF can promote growth of myeloid cells, including malignant cells, in vitro and similar effects may be seen on some non-myeloid cells in vitro.
Pharmacokinetic properties
Absorption
After subcutaneous injection of efbemalenograstim alfa, the peak serum concentration of efbemalenograstim alfa occurs at 36 hours [min-max: 6-96 hours] after dosing and serum concentrations of efbemalenograstim alfa are maintained during the period of neutropenia after myelosuppressive chemotherapy.
Distribution
The apparent volume of distribution ranges from 395 to 5679 mL/kg.
Biotransformation
Efbemalenograstim alfa is expected to be metabolized into small peptides by catabolic pathways.
Elimination
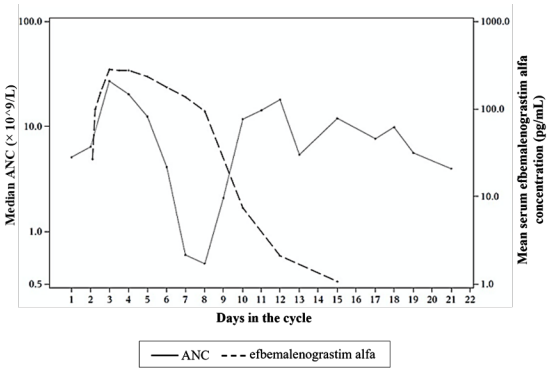
Efbemalenograstim alfa appears to be mainly eliminated by neutrophil-mediated clearance, which becomes saturated at higher doses. Consistent with a self-regulating clearance mechanism, the serum concentration of efbemalenograstim alfa declines rapidly at the onset of neutrophil recovery (see Figure 1). The half-life ranged from 19 to 84 hours after subcutaneous injection.
Linearity/non-linearity
Efbemalenograstim alfa exhibited non-linearity and time-dependent pharmacokinetics over the dose range of 30 to 360 mcg/kg.
Figure 1. Profile of median efbemalenograstim alfa serum concentration and Absolute Neutrophil Count (ANC) in chemotherapy treated patients after a single 320 mcg/kg injection:
Due to the neutrophil-mediated clearance mechanism, the pharmacokinetics of efbemalenograstim alfa is not expected to be affected by renal or hepatic impairment.
Elderly
Limited data indicate that the pharmacokinetics of efbemalenograstim alfa in elderly subjects (>65 years) is similar to that in adults.
Paediatric population
There are no data available on the pharmacokinetics of efbemalenograstim alfa in children.
Preclinical safety data
Preclinical data from conventional studies of repeated dose toxicity revealed the expected pharmacological effects including increases in leukocyte count, myeloid hyperplasia in bone marrow, extramedullary haematopoiesis and splenic enlargement.
There were no adverse events observed in offspring from pregnant rats or rabbits given efbemalenograstim alfa subcutaneously at cumulative doses approximately 2.6 and 0.7 times, respectively, the recommended human dose. Similar studies of other G-CSF products in rabbits have shown embryo/foetal toxicity (embryo loss) at cumulative doses approximately 4 times the recommended human dose, which were not seen when pregnant rabbits were exposed to the recommended human dose. Studies in rats indicated that reproductive performance, fertility, oestrous cycling, days between pairing and coitus, and intrauterine survival were unaffected by efbemalenograstim alfa given subcutaneously. The relevance of these findings for humans is not known.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.