Elagolix
Chemical formula: C₃₂H₃₀F₅N₃O₅ Molecular mass: 631.6 g/mol PubChem compound: 11250647
Pregnancy
Risk Summary
Use of elagolix is contraindicated in pregnant women. Exposure to elagolix early in pregnancy may increase the risk of early pregnancy loss. Discontinue elagolix if pregnancy occurs during treatment.
The limited human data with the use of elagolix in pregnant women are insufficient to determine whether there is a risk for major birth defects or miscarriage. Although two cases of congenital malformations were reported in clinical trials with elagolix, no pattern was identified and miscarriages were reported at a similar incidence across treatment groups (see Data).
When pregnant rats and rabbits were orally dosed with elagolix during the period of organogenesis, postimplantation loss was observed in pregnant rats at doses 20 times the maximum recommended human dose (MRHD). Spontaneous abortion and total litter loss was observed in rabbits at doses 7 and 12 times the MRHD. There were no structural abnormalities in the fetuses at exposures up to 40 and 12 times the MRHD for the rat and rabbit, respectively (see Data).
Data
Human Data
There were 49 pregnancies reported in clinical trials of more than 3,500 women (of whom more than 2,000 had endometriosis) treated with elagolix for up to 12 months. These pregnancies occurred while the women were receiving elagolix or within 30 days after stopping elagolix. Among these 49 pregnancies, two major congenital malformations were reported. In one case of infant cleft palate, the mother was treated with elagolix 150 mg daily and the estimated fetal exposure to elagolix occurred during the first 30 days of pregnancy. In one case of infant tracheoesophageal fistula, the mother was treated with elagolix 150 mg daily and the estimated fetal exposure to elagolix occurred during the first 15 days of pregnancy.
Among these 49 pregnancies, there were five cases of spontaneous abortion (miscarriage) compared to five cases among the 20 pregnancies that occurred in more than 1100 women treated with placebo. Although the duration of fetal exposure was limited in elagolix clinical trials, there were no apparent decreases in birth weights associated with elagolix in comparison to placebo.
Animal Data
Embryofetal development studies were conducted in the rat and rabbit. Elagolix was administered by oral gavage to pregnant rats (25 animals/dose) at doses of 0, 300, 600 and 1200 mg/kg/day and to rabbits (20 animals/dose) at doses of 0, 100, 150, and 200 mg/kg/day, during the period of organogenesis (gestation day 6-17 in the rat and gestation day 7-20 in the rabbit).
In rats, maternal toxicity was present at all doses and included six deaths and decreases in body weight gain and food consumption. Increased postimplantation losses were present in the mid dose group, which was 20 times the MRHD based on AUC. In rabbits, three spontaneous abortions and a single total litter loss were observed at the highest, maternally toxic dose, which was 12 times the MRHD based on AUC. A single total litter loss occurred at a lower non-maternally toxic dose of 150 mg/kg/day, which was 7 times the MRHD.
No fetal malformations were present at any dose level tested in either species even in the presence of maternal toxicity. At the highest doses tested, the exposure margins were 40 and 12 times the MRHD for the rat and rabbit, respectively. However, because elagolix binds poorly to the rat gonadotropin-releasing hormone (GnRH) receptor (~1000 fold less than to the human GnRH receptor), the rat study is unlikely to identify pharmacologically mediated effects of elagolix on embryofetal development. The rat study is still expected to provide information on potential non-target-related effects of elagolix.
In a pre- and postnatal development study in rats, elagolix was given in the diet to achieve doses of 0, 100 and 300 mg/kg/day (25 per dose group) from gestation day 6 to lactation day 20. There was no evidence of maternal toxicity. At the highest dose, two dams had total litter loss, and one failed to deliver. Pup survival was decreased from birth to postnatal day 4. Pups had lower birth weights and lower body weight gains were observed throughout the pre-weaning period at 300 mg/kg/day. Smaller body size and effect on startle response were associated with lower pup weights at 300 mg/kg/day. Post-weaning growth, development and behavioral endpoints were unaffected.
Maternal plasma concentrations in rats on lactation day 21 at 100 and 300 mg/kg/day (47 and 125 ng/mL) were 0.06-fold and 0.16-fold the maximal elagolix concentration (Cmax) in humans at the MRHD. Because the exposures achieved in rats were much lower than the human MRHD, this study is not predictive of potentially higher lactational exposure in humans.
Nursing mothers
Risk Summary
There is no information on the presence of elagolix or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. There are no adequate animal data on the excretion of elagolix in milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for elagolix and any potential adverse effects on the breastfed child from elagolix.
Data
There are no adequate animal data on excretion of elagolix in milk.
Carcinogenesis, mutagenesis and fertility
Two-year carcinogenicity studies conducted in mice (50, 150, or 500 mg/kg/day) and rats (150, 300, or 800 mg/kg/day) that administered elagolix by the dietary route revealed no increase in tumors in mice at up to 19-fold the MRHD based on AUC. In the rat, there was an increase in thyroid (male and female) and liver (males only) tumors at the high dose (12 to 13-fold the MRHD). The rat tumors were likely species-specific and of negligible relevance to humans.
Elagolix was not genotoxic or mutagenic in a battery of tests, including the in vitro bacterial reverse mutation assay, the in vitro mammalian cell forward mutation assay at the thymidine kinase (TK+/-) locus in L5178Y mouse lymphoma cells, and the in vivo mouse micronucleus assay.
In a fertility study conducted in the rat, there was no effect of elagolix on fertility at any dose (50, 150, or 300 mg/kg/day). Based on AUC, the exposure multiple for the MRHD in women compared to the highest dose of 300 mg/kg/day in female rats is approximately 5-fold. However, because elagolix has low affinity for the GnRH receptor in the rat, and because effects on fertility are most likely to be mediated via the GnRH receptor, these data have low relevance to humans.
Adverse reactions
The following serious adverse reactions are discussed elsewhere in labeling:
- Bone loss
- Change in menstrual bleeding pattern and reduced ability to recognize pregnancy
- Suicidal ideation, suicidal behavior, and exacerbation of mood disorders
- Hepatic transaminase elevations
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of elagolix was evaluated in two six-month, randomized, double-blind, placebo-controlled clinical trials [EM-1 (NCT01620528) and EM-2 (NCT01931670)] in which a total of 952 adult women with moderate to severe pain associated with endometriosis were treated with elagolix (475 with 150 mg once daily and 477 with 200 mg twice daily) and 734 were treated with placebo. The population age range was 18-49 years old. Women who completed six months of treatment and met eligibility criteria continued treatment in two uncontrolled, blinded six-month extension trials [EM-3 (NCT01760954) and EM-4 (NCT02143713)], for a total treatment duration of up to 12 months.
Serious Adverse Events
Overall, the most common serious adverse events reported for subjects treated with elagolix in the two placebo-controlled clinical trials (Studies EM-1 and EM-2) included appendicitis (0.3%), abdominal pain (0.2%), and back pain (0.2%). In these trials, 0.2% of subjects treated with elagolix 150 mg once daily and 0.2% of subjects treated with elagolix 200 mg twice daily discontinued therapy due to serious adverse reactions compared to 0.5% of those given placebo.
Adverse Reactions Leading to Study Discontinuation
In the two placebo-controlled clinical trials (Studies EM-1 and EM-2), 5.5% of subjects treated with elagolix 150 mg once daily and 9.6% of subjects treated with elagolix 200 mg twice daily discontinued therapy due to adverse reactions compared to 6.0% of those given placebo. Discontinuations were most commonly due to hot flushes or night sweats (1.1% with 150 mg once daily and 2.5% with 200 mg twice daily) and nausea (0.8% with 150 mg once daily and 1.5% with 200 mg twice daily) and were dose-related. The majority of discontinuations due to hot flushes or night sweats (10 of 17, 59%) and nausea (7 of 11, 64%) occurred within the first 2 months of therapy.
In the two extension trials (Studies EM-3 and EM-4), discontinuations were most commonly due to decreased BMD and were dose-related. In these trials, 0.3% of subjects treated with elagolix 150 mg once daily and 3.6% of subjects treated with elagolix 200 mg twice daily discontinued therapy due to decreased BMD.
Common Adverse Reactions
Adverse reactions reported in ≥5% of women in the two placebo-controlled trials in either elagolix dose group and at a greater frequency than placebo are noted in the following table.
Table 1. Percentage of Subjects in Studies EM-1 and EM-2 with Treatment-Emergent Adverse Reactions Occurring in at Least 5% of Subjects (either elagolix Dose Group) and at a Greater Incidence than with Placebo:
| Elagolix 150 mg Once Daily N=475 | Elagolix 200 mg Twice Daily N=477 | Placebo N=734 | |
|---|---|---|---|
| % | % | % | |
| Hot Flush | 24 | 46 | 9 |
| Headache | 17 | 20 | 12 |
| Nausea | 11 | 16 | 13 |
| Insomnia | 6 | 9 | 3 |
| Mood altered, mood swings | 6 | 5 | 3 |
| Amenorrhea | 4 | 7 | <1 |
| Depressed mood, depression, depressive symptoms and/or tearfulness | 3 | 6 | 2 |
| Anxiety | 3 | 5 | 3 |
| Arthralgia | 3 | 5 | 3 |
Less Common Adverse Reactions
In Study EM-1 and Study EM-2, adverse reactions reported in ≥3% and <5% in either elagolix dose group and greater than placebo included: decreased libido, diarrhea, abdominal pain, weight gain, dizziness, constipation and irritability.
The most commonly reported adverse reactions in the extension trials (EM-3 and EM-4) were similar to those in the placebo-controlled trials.
Bone Loss
The effect of elagolix on BMD was assessed by dual-energy X-ray absorptiometry (DXA).
In Studies EM-1 and EM-2, there was a dose-dependent decrease in BMD in elagolix-treated subjects compared to an increase in placebo-treated subjects.
In Study EM-1, compared to placebo, the mean change from baseline in lumbar spine BMD at 6 months was -0.9% (95% CI: -1.3, -0.4) with elagolix 150 mg once daily and -3.1% (95% CI: -3.6, -2.6) with elagolix 200 mg twice daily (Table 2). The percentage of subjects with greater than 8% BMD decrease in lumbar spine, total hip or femoral neck at any time point during the placebo-controlled treatment period was 2% with elagolix 150 mg once daily, 7% with elagolix 200 mg twice daily and <1% with placebo. In the blinded extension Study EM-3, continued bone loss was observed with 12 months of continuous treatment with elagolix. The percentage of subjects with greater than 8% BMD decrease in lumbar spine, total hip or femoral neck at any time point during the extension treatment period was 8% with continuous elagolix 150 mg once daily and 21% with continuous elagolix 200 mg twice daily.
In Study EM-2, compared to placebo, the mean change from baseline in lumbar spine BMD at 6 months was -1.3% (95% CI: -1.8, -0.8) with elagolix 150 mg once daily and -3.0% (95% CI: -3.5, -2.6) with elagolix 200 mg twice daily (Table 2). The percentage of subjects with greater than 8% BMD decrease in lumbar spine, total hip or femoral neck at any time point during the placebo-controlled treatment period was <1% with elagolix 150 mg once daily, 6% with elagolix 200 mg twice daily and 0% with placebo. In the blinded extension Study EM-4, continued bone loss was observed with 12 months of continuous treatment with elagolix. The percentage of subjects with greater than 8% BMD decrease in lumbar spine, total hip or femoral neck at any time point during the extension treatment period was 2% with continuous elagolix 150 mg once daily and 21% with continuous elagolix 200 mg twice daily.
Table 2. Percent Change from Baseline in Lumbar Spine BMD at Month 6:
| Elagolix 150 mg Once Daily | Elagolix 200 mg Twice Daily | Placebo | |
|---|---|---|---|
| EM-1 | |||
| N | 183 | 180 | 277 |
| Percent Change from Baseline, % | -0.3 | -2.6 | 0.5 |
| Treatment Difference, % (95% CI) | -0.9 (-1.3, -0.4) | -3.1 (-3.6, -2.6) | |
| EM-2 | |||
| N | 174 | 183 | 271 |
| Percent Change from Baseline, % | -0.7 | -2.5 | 0.6 |
| Treatment Difference, % (95% CI) | -1.3 (-1.8, -0.8) | -3.0 (-3.5, -2.6) | |
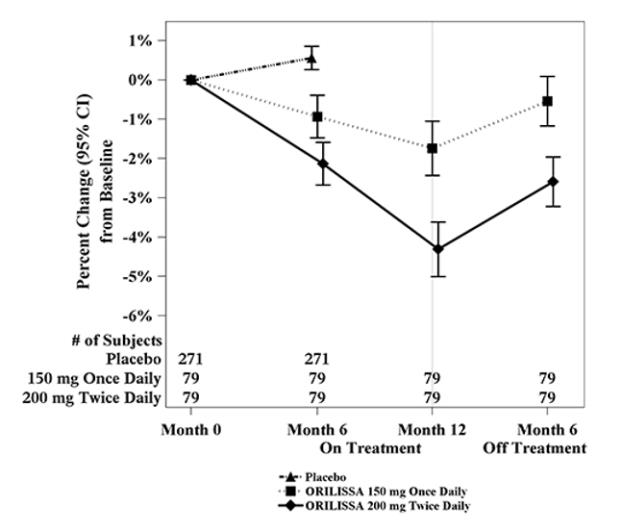
To assess for recovery, the change in lumbar spine BMD over time was analyzed for subjects who received continuous treatment with elagolix 150 mg once daily or elagolix 200 mg twice daily for up to 12 months and who were then followed after cessation of therapy for an additional 6 months. Partial recovery of BMD was seen in these subjects (Figure 1).
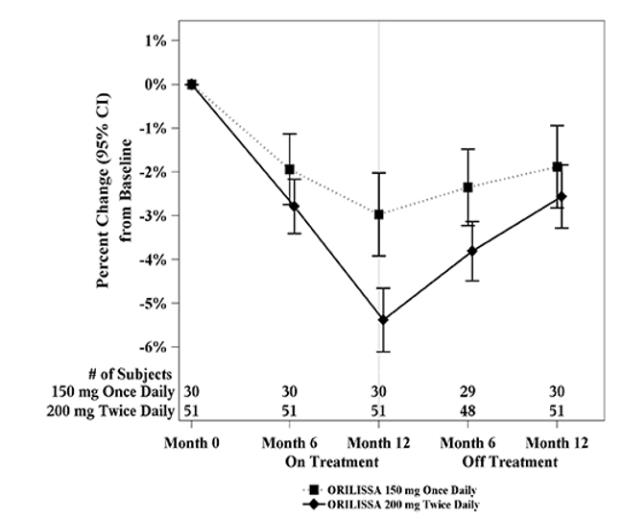
In Study EM-3, if a subject had BMD loss of more than 1.5% at the lumbar spine or more than 2.5% at the total hip at the end of treatment, follow-up DXA was required after 6 months off-treatment. In Study EM-4, all subjects were required to have a follow-up DXA 6 months off treatment regardless of change in BMD and if a subject had BMD loss of more than 1.5% at the lumbar spine or more than 2.5% at the total hip after 6 months off treatment, follow-up DXA was required after 12 months off-treatment. Figure 2 shows the change in lumbar spine BMD for the subjects in Study EM-2/EM-4 who completed 12 months of treatment with elagolix and who had a follow-up DXA 12-months off treatment.
Figure 1. Percent Change from Baseline in Lumbar Spine BMD in Subjects Who Received 12 Months of elagolix and Had Follow-up BMD 6 Months off Therapy in Studies EM-2/EM-4:
Figure 2. Percent Change from Baseline in Lumbar Spine BMD in Subjects Who Received 12 Months of elagolix and Had Follow-up BMD 12 Months off Therapy in Studies EM-2/EM-4:
Suicidal Ideation, Suicidal Behavior and Exacerbation of Mood Disorders
In the placebo-controlled trials (Studies EM-1 and EM-2), elagolix was associated with adverse mood changes (see Table 1 and Table 3), particularly in those with a history of depression.
Table 3. Suicidal Ideation and Suicidal Behavior in Studies EM-1 and EM-2:
| Elagolix | Placebo (N=734) n (%) | ||
|---|---|---|---|
| Adverse Reactions | 150 mg Once Daily (N=475) n (%) | 200 mg Twice Daily (N=477) n (%) | |
| Completed suicide | 1 (0.2) | 0 | 0 |
| Suicidal ideation | 1 (0.2) | 1 (0.2) | 0 |
A 44-year-old woman received 31 days of elagolix 150 mg once daily then completed suicide 2 days after elagolix discontinuation. She had no relevant past medical history; life stressors were noted.
Among the 2090 subjects exposed to elagolix in the endometriosis Phase 2 and Phase 3 studies, there were four reports of suicidal ideation. In addition to the two subjects in Table 3, there were two additional reports of suicidal ideation: one subject in EM-3 (150 mg once daily) and one in a Phase 2 study (75 mg once daily, an unapproved dose). Three of these subjects had a history of depression. Two subjects discontinued elagolix and two completed the clinical trial treatment periods.
Hepatic Transaminase Elevations
In the placebo-controlled clinical trials (Studies EM-1 and EM-2), dose-dependent asymptomatic elevations of serum ALT to at least 3-times the upper limit of the reference range occurred during treatment with elagolix (150 mg once daily – 1/450, 0.2%; 200 mg twice daily – 5/443, 1.1%; placebo – 1/696, 0.1%). Similar increases were seen in the extension trials (Studies EM-3 and EM-4).
Changes in Lipid Parameters
Dose-dependent increases in total cholesterol, low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and serum triglycerides were noted during elagolix treatment in EM-1 and EM-2. In EM-1 and EM-2, 12% and 1% of subjects with mildly elevated LDL-C (130-159 mg/dL) at baseline had an increase in LDL-C concentrations to 190 mg/dL or higher during treatment with elagolix and placebo, respectively. In EM-1 and EM-2, 4% and 1% of subjects with mildly elevated serum triglycerides (150-300 mg/dL) at baseline had an increase in serum triglycerides to at least 500 mg/dL during treatment with elagolix and placebo, respectively. The highest measured serum triglyceride concentration during treatment with elagolix was 982 mg/dL.
Table 4. Mean Change and Maximum Increase from Baseline in Serum Lipids in Studies EM-1 and EM-2:
| Elagolix 150 mg Once Daily N=475 | Elagolix 200 mg Twice Daily N=477 | Placebo N=734 | |
|---|---|---|---|
| LDL-C (mg/dL) | |||
| Mean change at Month 6 | 5 | 13 | -3 |
| Maximum increase during Treatment Period | 137 | 107 | 122 |
| HDL-C (mg/dL) | |||
| Mean change at Month 6 | 2 | 4 | 1 |
| Maximum increase during Treatment Period | 43 | 52 | 45 |
| Triglycerides (mg/dL) | |||
| Mean change at Month 6 | <1 | 11 | -3 |
| Maximum increase during Treatment Period | 624 | 484 | 440 |
Lipid increases occurred within 1 to 2 months after the start of elagolix and remained stable thereafter over 12 months.
Hypersensitivity Reactions
In Studies EM-1 and EM-2, non-serious hypersensitivity reactions including rash occurred in 5.8% of elagolix treated-subjects and 6.1% of placebo-treated subjects. These events led to study drug discontinuation in 0.4% of elagolix-treated subjects and 0.5% of placebo-treated subjects.
Endometrial Effects
Endometrial biopsies were performed in subjects in Study EM-1 and its extension at Month 6 and Month 12. These biopsies showed a dose-dependent decrease in proliferative and secretory biopsy patterns and an increase in quiescent/minimally stimulated biopsy patterns. There were no abnormal biopsy findings on treatment, such as endometrial hyperplasia or cancer.
Based on transvaginal ultrasound, during the course of a 3-menstrual cycle study in healthy women, elagolix 150 mg once daily and 200 mg twice daily resulted in a dose-dependent decrease from baseline in mean endometrial thickness.
Effects on menstrual bleeding patterns
The effects of elagolix on menstrual bleeding were evaluated for up to 12 months using an electronic daily diary where subjects classified their flow of menstrual bleeding (if present in the last 24 hours) as spotting, light, medium, or heavy. Elagolix led to a dose-dependent reduction in mean number of bleeding and spotting days and bleeding intensity in those subjects who reported menstrual bleeding.
Table 5. Mean Bleeding/Spotting Days and Mean Intensity Scores at Month 3:
| Elagolix 150mg Once Daily | Elagolix 200mg Twice Daily | Placebo | ||||
|---|---|---|---|---|---|---|
| Baseline | Month 3 | Baseline | Month 3 | Baseline | Month 3 | |
| Mean bleeding/ spotting days in prior 28 days | 5.3 | 2.8 | 5.7 | 0.8 | 5.4 | 4.6 |
| Mean Intensity scorea | 2.6 | 2.2 | 2.5 | 2.0 | 2.6 | 2.4 |
a Intensity for subjects who reported at least 1 day of bleeding or spotting during 28 day interval. Scale ranges from 1 to 4, 1 = spotting, 2 = light, 3 = medium, 4 = heavy
Elagolix also demonstrated a dose-dependent increase in the percentage of women with amenorrhea (defined as no bleeding or spotting in a 56-day interval) over the treatment period. The incidence of amenorrhea during the first six months of treatment ranged from 6-17% for elagolix 150 mg once daily, 13-52% for elagolix 200 mg twice daily and less than 1% for placebo. During the second 6 months of treatment, the incidence of amenorrhea ranged from 11-15% for elagolix 150 mg once daily and 46-57% for elagolix 200 mg twice daily.
After 6 months of therapy with elagolix 150 mg once daily, resumption of menses after stopping treatment was reported by 59%, 87% and 95% of women within 1, 2, and 6 months, respectively. After 6 months of therapy with elagolix 200 mg twice daily, resumption of menses after stopping treatment was reported by 60%, 88%, and 97% of women within 1, 2, and 6 months, respectively.
After 12 months of therapy with elagolix 150 mg once daily resumption of menses after stopping treatment was reported by 77%, 95% and 98% of women within 1, 2, and 6 months respectively. After 12 months of therapy with elagolix 200 mg twice daily resumption of menses after stopping treatment was reported by 55%, 91% and 96% of women within 1, 2, and 6 months respectively.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.