Emicizumab
Mechanism of action
Emicizumab is a humanized monoclonal modified immunoglobulin G4 (IgG4) antibody with a bispecific antibody structure.
Emicizumab bridges activated factor IX and factor X to restore the function of missing activated factor VIII that is needed for effective haemostasis.
Emicizumab has no structural relationship or sequence homology to factor VIII and, as such, does not induce or enhance the development of direct inhibitors to factor VIII.
Pharmacodynamic properties
Pharmacodynamics
Prophylactic therapy with emicizumab shortens the aPTT and increases the reported factor VIII activity (using a chromogenic assay with human coagulation factors). These two pharmacodynamic markers do not reflect the true haemostatic effect of emicizumab in vivo (aPTT is overly shortened and reported factor VIII activity may be overestimated) but provide a relative indication of the pro-coagulant effect of emicizumab.
Pharmacokinetic properties
The pharmacokinetics of emicizumab was determined via non-compartmental analysis in healthy subjects and using a population pharmacokinetic analysis on a database composed of 389 patients with haemophilia A.
Absorption
Following subcutaneous administration in haemophilia A patients, the absorption half-life was 1.6 days.
Following multiple subcutaneous administrations of 3 mg/kg once weekly for the first 4 weeks in haemophilia A patients, mean (±SD) trough plasma concentrations of emicizumab achieved 52.6±13.6 μg/mL at Week 5.
The predicted mean (±SD) Ctrough, and Cmax and ratios of Cmax/Ctrough at steady-state for the recommended maintenance doses of 1.5 mg/kg once weekly, 3 mg/kg every two weeks or 6 mg/kg every four weeks are shown in the following table.
Mean (± SD) steady-state emicizumab concentrations:
| Maintenance dose | |||
|---|---|---|---|
| Parameters | 1.5 mg/kg QW | 3 mg/kg Q2W | 6 mg/kg Q4W |
| Cmax,ss (µg/mL) | 54.9 ± 15.9 | 58.1 ± 16.5 | 66.8 ± 17.7 |
| Cavg,ss (µg/mL) | 53.5 ± 15.7 | 53.5 ± 15.7 | 53.5 ± 15.7 |
| Ctrough,ss (µg/mL) | 51.1 ± 15.3 | 46.7 ± 16.9 | 38.3 ± 14.3 |
| Cmax/Ctrough ratio | 1.08 ± 0.03 | 1.26 ± 0.12 | 1.85 ± 0.46 |
Cavg,ss = average concentration at steady state; Cmax,ss = maximum plasma concentration at steady state; Ctrough,ss = trough concentration at steady state; QW = once weekly; Q2W = every two weeks; Q4W = every four weeks.
Pharmacokinetic parameters derived from the population PK model.
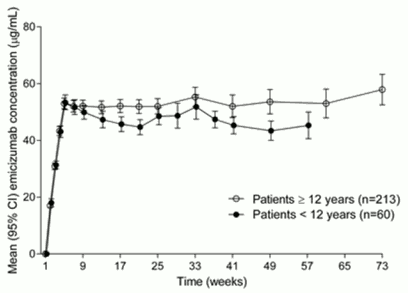
Similar PK profiles were observed following once weekly dosing (3 mg/kg/week for 4 weeks followed by 1.5 mg/kg/week) in adults/adolescents (≥12 years) and children (<12 years) (see Figure 1).
Figure 1. Mean (±95% CI) plasma emicizumab concentration versus time profiles for patients ≥12 years (studies HAVEN 1 and HAVEN 3) compared with patients <12 years (study HAVEN 2):
In healthy subjects, the absolute bioavailability following subcutaneous administration of 1 mg/kg was between 80.4% and 93.1% depending on the injection site. Similar pharmacokinetic profiles were observed following subcutaneous administration in the abdomen, upper arm, and thigh. Emicizumab can be administered interchangeably at these anatomical sites.
Distribution
Following a single intravenous dose of 0.25 mg/kg emicizumab in healthy subjects, the volume of distribution at steady state was 106 mL/kg (i.e. 7.4 L for a 70-kg adult).
The apparent volume of distribution (V/F), estimated from the population PK analysis, in haemophilia A patients following multiple subcutaneous doses of emicizumab was 10.4 L.
Metabolism
The metabolism of emicizumab has not been studied. IgG antibodies are mainly catabolised by lysosomal proteolysis and then eliminated from or reused by the body.
Elimination
Following intravenous administration of 0.25 mg/kg in healthy subjects, the total clearance of emicizumab was 3.26 mL/kg/day (i.e. 0.228 L/d for a 70-kg adult) and the mean terminal half-life was 26.7 days.
Following single subcutaneous injection in healthy subjects, the elimination half-life was approximately 4 to 5 weeks.
Following multiple subcutaneous injections in haemophilia A patients, the apparent clearance was 0.272 L/day and the elimination apparent half-life was 26.8 days.
Dose linearity
Emicizumab exhibited dose-proportional pharmacokinetics in patients with haemophilia A after the first dose of emicizumab over a dose range from 0.3 to 6 mg/kg. The exposure (Cavg,ss) of multiple doses is comparable between 1.5 mg/kg every week, 3mg/kg every 2 weeks and 6mg/kg dose every 4 weeks.
Special populations
Paediatric
The effect of age on the pharmacokinetics of emicizumab was assessed in a population pharmacokinetic analysis which included 5 infants (≥1 month to <2 years), 55 children (less than 12 years) and 50 adolescents (12 to <18 years) with haemophilia A. Age did not affect the pharmacokinetics of emicizumab in paediatric patients.
Elderly
The effect of age on the pharmacokinetics of emicizumab was assessed in a population pharmacokinetic analysis which included thirteen subjects aged 65 years and older (no subjects were older than 77 years of age). Relative bioavailability decreased with older age, but no clinically important differences were observed in the pharmacokinetics of emicizumab between subjects <65 years and subjects ≥65 years.
Race
Population pharmacokinetics analyses in patients with haemophilia A showed that race did not affect the pharmacokinetics of emicizumab. No dose adjustment is required for this demographic factor.
Gender
Data in female patients are too limited for conclusion.
Renal impairment
No dedicated studies of the effect of renal impairment on the pharmacokinetics of emicizumab have been conducted.
Most of the patients with hemophilia A in the population pharmacokinetic analysis had normal renal function (N=332; creatinine clearance [CLcr] ≥90 mL/min) or mild renal impairment (N=27; CLcr of 60-89 mL/min). Mild renal impairment did not affect the pharmacokinetics of emicizumab. There are limited data available on the use of emicizumab in patients with moderate renal impairment (only 2 patients with CLcr of 30-59 mL/min) and no data in patients with severe renal impairment. The impact of moderate and severe renal impairment on the pharmacokinetics of emicizumab cannot be concluded.
Emicizumab is a monoclonal antibody and is cleared via catabolism rather than renal excretion and a change in dose is not expected to be required for patients with renal impairment.
Hepatic impairment
No dedicated studies on the effect of hepatic impairment on the pharmacokinetics of emicizumab have been conducted. Most of the patients with haemophilia A in the population pharmacokinetic analysis had normal hepatic function (bilirubin and AST ≤ ULN, N=300) or mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin from 1.0 to 1.5 × ULN and any AST, N=51). Only 6 patients had moderate hepatic impairment (1.5 × ULN < bilirubin ≤3 × ULN and any AST). Mild hepatic impairment did not affect the pharmacokinetics of emicizumab. The safety and efficacy of emicizumab have not been specifically tested in patients with hepatic impairment. Patients with mild and moderate hepatic impairment were included in clinical trials. No data are available on the use of emicizumab in patients with severe hepatic impairment.
Emicizumab is a monoclonal antibody and cleared via catabolism rather than hepatic metabolism and a change in dose is not expected to be required for patients with hepatic impairment.
Other special populations
Modelling shows that less frequent dosing in patients with hypoalbuminemia and low body weight for their age results in lower emicizumab exposures; simulations indicate that these patients would still benefit from clinically meaningful bleed control. No patients with such characteristics were enrolled in clinical trials.
Preclinical safety data
Preclinical data reveal no special hazards for humans based on studies of acute and repeated dose toxicity, including safety pharmacology endpoints and endpoints for reproductive toxicity.
Fertility
Emicizumab did not cause any changes in the reproductive organs of male or female cynomolgus monkeys up to the highest tested dose of 30 mg/kg/week (equivalent to 11 times the human exposure at the highest dose of 3 mg/kg/week, based on AUC).
Teratogenicity
No data are available with respect to potential side effects of emicizumab on embryo-foetal development.
Injection site reactions
Reversible hemorrhage, perivascular mononuclear cell infiltration, degeneration/necrosis of subcutis and swelling of endothelium in the subcutis was noted in animals after subcutaneous injection.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.