Human normal immunoglobulin G
Pharmacodynamic properties
Human normal immunoglobulin contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against infectious agents.
Human normal immunoglobulin contains the IgG antibodies present in the normal population. It is usually prepared from pooled plasma from not fewer than 1,000 donors. It has a distribution of immunoglobulin G subclasses closely proportional to that in native human plasma.
In immunodeficiency, adequate doses of human normal immunoglobulin may restore abnormally low immunoglobulin G antibody levels to the normal range and thus help against infections. The mechanism of action in indications other than replacement therapy is not fully elucidated, but includes immunomodulatory effects.
Pharmacokinetic properties
SC administration
Absorption and Distribution
Following subcutaneous administration of human normal immunoglobulin G, peak serum levels are achieved after approximately 2 days.
Elimination
IgG and IgG-complexes are broken down in cells of the reticuloendothelial system.
PID
In a clinical phase III trial with IgG (n=46), the subjects achieved sustained trough levels (median 8.1 g/l) over a period of 29 weeks when receiving median weekly doses of 0.06 to 0.24 g/kgbw.
Simulations by empirical Population Pharmacokinetic models suggested that comparable IgG exposure levels (AUC0-14days, Cmin,14days) may be obtained if IgG is administered subcutaneously every two weeks using double the weekly dose during maintenance therapy.
These simulations further suggested that comparable serum IgG trough levels can be achieved when the weekly maintenance dose is administered in proportional amounts more frequently than once a week (e.g. 2 times per week, 3 times per week, 5 times per week or daily).
Simulation of 2-3 missed daily doses resulted in a median serum IgG level decrease of ≤4% compared to consistent daily dosing. By replacing the missed doses when daily dosing was resumed, the median concentration profile recovered within 2 to 3 days. However, if missed doses were not replaced when dosing was resumed, it took up to 5-6 weeks for the IgG trough levels to return to steady-state.
Paediatric population
No differences were seen in the pharmacokinetic parameters between adult and paediatric PID study patients.
Elderly
No overall differences in the pharmacokinetic parameters were observed between PID subjects >65 years and subjects 18 to 65 years of age.
CIDP
In the PATH study, subjects (n=172) achieved sustained trough levels over a period of 24 weeks when receiving weekly doses of 0.2 g/kg bw and 0.4 g/kg bw, respectively. The mean (SD) IgG trough concentration after IgG treatment in the 0.4 g/kg bw group was 20.4 (3.24) g/l and 15.4 (3.06) g/l in the 0.2 g/kg bw group. Simulations with population-pharmacokinetic models in the PATH study suggest that a comparable IgG exposure (Cmax, AUC0-14days, Cmin,14 days) is achieved when the double weekly dose is administered every two weeks in the CIDP subjects. These simulations further suggest that a comparable IgG exposure is correspondingly achieved when the weekly maintenance dose of IgG is divided in several, more frequent doses (2 to 7 times per week) in the CIDP patients' population.
Paediatric population
Human normal immunoglobulin G has not been evaluated in clinical studies in paediatric patients with CIDP who are under the age of 18.
Elderly
No overall differences in the pharmacokinetic parameters were observed between CIDP subjects >65 years and subjects 18 to 65 years of age.
IV administration
Treatment of Primary Humoral Immunodeficiency (PI)
In the PI study, 50 pediatric and adult subjects underwent pharmacokinetic assessments. Subjects received infusions of IgG (200 to 800 mg/kg body weight) every 3 or 4 weeks for 12 months. Blood samples for PK study were collected between the 7th and 9th IgG infusion, depending on the individual treatment schedule.
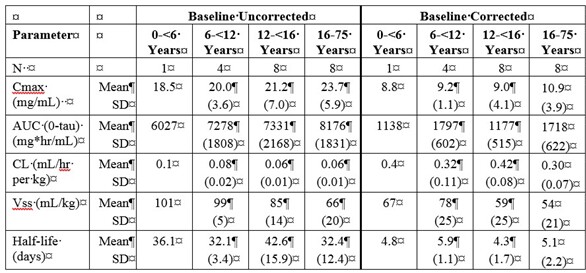
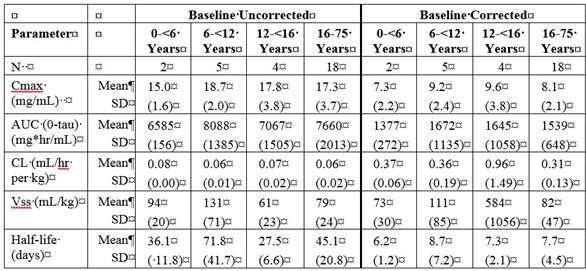
Table 1a and 1b summarize the pharmacokinetic parameters of IgG, based on serum concentrations of total IgG, in subjects receiving infusions every 3, or 4 weeks, respectively.
Table 1. PI Study – Pharmacokinetic Parameters of IgG in Subjects:
a) PK Parameters: IgG Arm: 3-weeks
b) PK Parameters: IgG Arm: 4-weeks
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
Pharmacokinetic studies with IgG have not been performed in adults with chronic ITP.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
Pharmacokinetic studies with IgG have not been performed in adult patients with CIDP.
The IgG trough levels were evaluated at each visit (Table 6) before the IgG infusion. There was the option of rescue treatment with two consecutive infusions of 2.0 g/kg IgG at 3-week intervals (±4 days) for all subjects in the 0.5 and 1.0 g/kg IgG arms who were either stable at Week 6 or deteriorated after Week 3 and before Week 18. The actual administered dose for the three dose arms was: 0.91 ± 0.4 (n=35), 1.24 ± 0.2 (n=69) and 1.97 ± 0.2 (n=38) g/kg for 0.5, 1 and 2g/kg, respectively. There were no major differences among the three dose arms in demographic characteristics and baseline IgG levels. The percentage of increase in mean IgG from baseline to the end of study assessment was 46% in the 0.5 g/kg arm, 57% in the 1.0 g/kg arm and 91% in the 2.0 g/kg arm (Table 6).
Table 6. Mean IgG trough levels in subjects with CIDP:
| IgG troughs (g/L) | IgG 0.5 g/kg | IgG 1.0 g/kg | IgG 2.0 g/kg | |
|---|---|---|---|---|
| Visit / Time point | (N=35) | (N=69) | (N=38) | |
| Visit 2 – Week 0 | Mean (SD) | 10.6 (3.1) | 10.5 (2.5) | 10.2 (3.0) |
| Visit 3 – Week 3 | Mean (SD) | 17.9 (3.5) | 17.1 (3.1) | 16.5 (3.3) |
| Visit 4 – Week 6 | Mean (SD) | 15.5 (3.0) | 16.5 (3.3) | 18.5 (4.0) |
| Visit 5 – Week 9 | Mean (SD) | 15.6 (3.3) | 16.5 (3.0) | 19.2 (4.4) |
| Visit 6 – Week 12 | Mean (SD) | 14.2 (2.6) | 16.8 (3.8) | 19.6 (4.3) |
| Visit 7 – Week 15 | Mean (SD) | 14.1 (2.7) | 16.2 (3.4) | 19.7 (4.4) |
| Visit 8 – Week 18 | Mean (SD) | 14.1 (2.3) | 15.9 (3.1) | 19.6 (5.2) |
| Visit 9 – Week 21 | Mean (SD) | 14.3 (2.3) | 16.0 (3.0) | 18.9 (3.5) |
| End of Study – Week 24 | Mean (SD) | 15.5 (3.6) | 16.5 (3.3) | 19.5 (4.6) |
IM administration
Peak levels of immunoglobulin G are obtained approximately two days after intramuscular injection of IgG.(16) The half-life of IgG in the circulation of individuals with normal IgG levels is 23 days.(17)
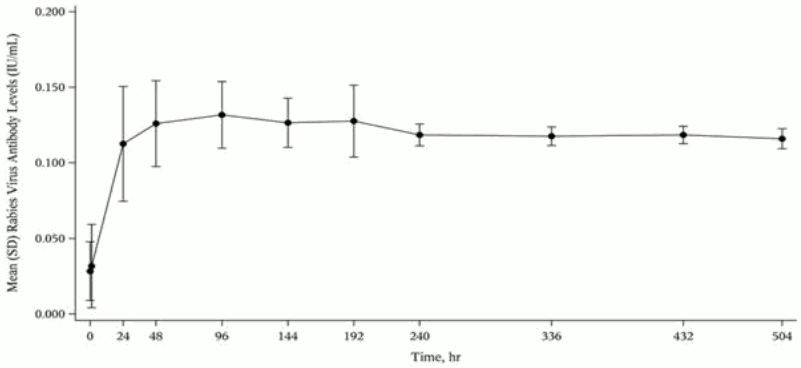
In a clinical study, 12 healthy human subjects received a 20 IU/kg intramuscular dose of IgG, (Rabies Immune Globulin (Human)), made using the same manufacturing process as IgG. Detectable passive rabies neutralizing antibody was present by 24 hours and persisted through the 21 day follow-up evaluation period. The figure below shows the mean levels of rabies virus antibodies in IU/mL across the 21 day evaluation period and indicates that the titer remains stable during this period.
Mean (Standard Deviation) Rabies Virus Antibody Levels (IU/mL) versus Time following a Single 20 IU/kg Dose of IgG (300 IU/mL) by Intramuscular Injection:
Preclinical safety data
Immunoglobulins are a normal constituent of the human body. L-proline is a physiological, nonessential amino acid.
The safety of IgG has been assessed in several preclinical studies, with particular reference to the excipient L-proline. Non-clinical data reveal no special risk for humans based on safety pharmacology and toxicity studies.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.