Insulin glulisine
Pharmacodynamic properties
Insulin glulisine is a recombinant human insulin analogue that is equipotent to regular human insulin. Insulin glulisine has a more rapid onset of action and a shorter duration of action than regular human insulin.
The primary activity of insulins and insulin analogues, including insulin glulisine, is regulation of glucose metabolism. Insulins lower blood glucose levels by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis and enhances protein synthesis.
Studies in healthy volunteers and patients with diabetes demonstrated that insulin glulisine is more rapid in onset of action and of shorter duration of action than regular human insulin when given subcutaneously. When insulin glulisine is injected subcutaneously, the glucose lowering activity will begin within 10-20 minutes. After intravenous administration, a faster onset and shorter duration of action, as well as a greater peak response were observed as compared with subcutaneous administration. The glucose-lowering activities of insulin glulisine and regular human insulin are equipotent when administered by intravenous route.
One unit of insulin glulisine has the same glucose-lowering activity as one unit of regular human insulin.
Pharmacokinetic properties
In insulin glulisine the replacement of the human insulin amino acid asparagine in position B3 by lysine and the lysine in position B29 by glutamic acid favours more rapid absorption. In a study with 18 male subjects with diabetes mellitus type 1, aged 21 to 50 years, insulin glulisine displays dose-proportionality for early, maximum and total exposure in the dose range 0.075 to 0.4 Units/kg.
Absorption and bioavailability
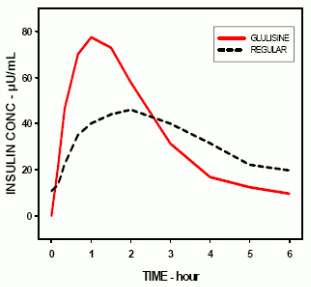
Pharmacokinetic profiles in healthy volunteers and diabetes patients (type 1 or 2) demonstrated that absorption of insulin glulisine was about twice as fast with a peak concentration approximately twice as high as compared to regular human insulin. In a study in patients with type 1 diabetes mellitus after subcutaneous administration of 0.15 Units/kg, for insulin glulisine the Tmax was 55 minutes and Cmax was 82 ± 1.3 μUnits/ml compared to a Tmax of 82 minutes and a Cmax of 46 ± 1.3 μUnits/ml for regular human insulin. The mean residence time of insulin glulisine was shorter (98 min) than for regular human insulin (161 min) (see figure).
Pharmacokinetic profile of insulin glulisine and regular human insulin in type 1 diabetes mellitus patients after a dose of 0.15 Units/kg:
In a study in patients with type 2 diabetes mellitus after subcutaneous administration of 0.2 Units/kg insulin glulisine, the Cmax was 91 μUnits/ml with the interquartile range from 78 to 104 μUnits/ml. When insulin glulisine was injected subcutaneously into abdomen, deltoid and thigh, the concentration-time profiles were similar with a slightly faster absorption when administered in the abdomen compared to the thigh. Absorption from deltoid sites was in-between. The absolute bioavailability (70%) of insulin glulisine was similar between injection sites and of low intra-subject variability (11% CV). Intravenous bolus administration of insulin glulisine resulted in a higher systemic exposure when compared to subcutaneous injection, with a Cmax approximately 40-fold higher.
Obesity
Another phase I study with insulin glulisine and insulin lispro in a non-diabetic population in 80 subjects with a wide range of body mass indices (18-46 kg/m²) has demonstrated that rapid absorption and total exposure is generally maintained across a wide range of body mass indices. The time to 10% of total INS exposure was reached earlier by approximately 5-6 min with insulin glulisine.
Distribution and elimination
The distribution and elimination of insulin glulisine and regular human insulin after intravenous administration is similar with volumes of distribution of 13 l and 22 l and half-lives of 13 and 18 minutes, respectively.
After subcutaneous administration, insulin glulisine is eliminated more rapidly than regular human insulin with an apparent half-life of 42 minutes compared to 86 minutes. In an across study analysis of insulin glulisine in either healthy subjects or subjects with type 1 or type 2 diabetes mellitus the apparent half-life ranged from 37 to 75 minutes (interquartile range). Insulin glulisine shows low plasma protein binding, similar to human insulin.
Special populations
Renal impairment
In a clinical study performed in non-diabetic subjects covering a wide range of renal function (CrCl >80 ml/min, 30-50 ml/min, <30 ml/min), the rapid-acting properties of insulin glulisine were generally maintained. However, insulin requirements may be reduced in the presence of renal impairment.
Hepatic impairment
The pharmacokinetic properties have not been investigated in patients with impaired liver function.
Elderly
Very limited pharmacokinetic data are available for elderly patients with diabetes mellitus.
Children and adolescents
The pharmacokinetic and pharmacodynamic properties of insulin glulisine were investigated in children (7-11 years) and adolescents (12-16 years) with type 1 diabetes mellitus. Insulin glulisine was rapidly absorbed in both age groups, with similar Tmax and Cmax as in adults. Administered immediately before a test meal, insulin glulisine provided better postprandial control than regular human insulin, as in adults. The glucose excursion (AUC 0-6h) was 641 mg.h.dl-1 for insulin glulisine and 801 mg.h.dl-1 for regular human insulin.
Preclinical safety data
Non-clinical data did not reveal toxicity findings others than those linked to the blood glucose lowering pharmacodynamic activity (hypoglycaemia), different from regular human insulin or of clinical relevance for humans.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.