Lipegfilgrastim
Mechanism of action
Lipegfilgrastim is a covalent conjugate of filgrastim with a single methoxy polyethylene glycol (PEG) molecule via a carbohydrate linker consisting of glycine, N-acetylneuraminic acid and N-acetylgalactosamine. The average molecular mass is approximately 39 kDa of which the protein moiety constitutes approximately 48%. Human G-CSF is a glycoprotein that regulates the production and release of functional neutrophils from the bone marrow. Filgrastim is an un-glycosylated recombinant methionyl human G-CSF. Lipegfilgrastim is a sustained duration form of filgrastim due to decreased renal clearance. Lipegfilgrastim binds to human the G-CSF receptor like filgrastim and pegfilgrastim.
Pharmacodynamic properties
Pharmacodynamic effects
Lipegfilgrastim and filgrastim induced a marked increase in peripheral blood neutrophil counts within 24 hours, with minor increases in monocytes and/or lymphocytes. These results suggest that the G-CSF moiety of lipegfilgrastim confers the expected activity of this growth factor: stimulation of proliferation of haematopoietic progenitor cells, differentiation into mature cells and release into the peripheral blood. This effect includes not only the neutrophil lineage but extends to other single lineage and multilineage progenitors and pluripotent haematopoietic stem cells. G-CSF also increases the antibacterial activities of neutrophils including the phagocytosis.
Pharmacokinetic properties
General
Healthy volunteers
In 3 studies (XM22-01, XM22-05, XM22-06) in healthy volunteers, the maximum blood concentration was reached after a median of 30 to 36 hours and the average terminal half-life ranged from approximately 32 to 62 hours after a single subcutaneous injection of 6 mg lipegfilgrastim.
After subcutaneous injection of 6 mg lipegfilgrastim at three different sites (upper arm, abdomen and thigh) in healthy volunteers, the bioavailability (peak concentration and area under the curve [AUC]) was lower after subcutaneous injection in the thigh compared to subcutaneous injection in the abdomen and in the upper arm. In this limited study XM22-06, bioavailability of lipegfilgrastim and observed differences among the injection sites were higher in male subjects compared to female subjects. Nevertheless, pharmacodynamic effects were similar and independent from gender and injection site.
Metabolism
Lipegfilgrastim is metabolised via intra- or extracellular degradation by proteolytic enzymes. Lipegfilgrastim is internalised by neutrophils (non-linear process), then degraded within the cell by endogenous proteolytic enzymes. The linear pathway is likely due to extracellular protein degradation by neutrophil elastase and other plasma proteases.
Drug interactions
In vitro data indicate that lipegfilgrastim is has little or no direct or immune system-mediated effects on CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A4/5 activity. Therefore, lipegfilgrastim is not likely to affect metabolism via human cytochrome P450 enzymes.
Special populations
Cancer patients
In 2 studies (XM22-02 and XM22-03) in patients with breast cancer receiving chemotherapy consisting of doxorubicin and docetaxel, mean maximum blood concentrations of 227 and 262 ng/ml were reached after median times to maximum concentration (tmax) of 44 and 48 hours. The mean terminal half-lives were approximately 29 and 31 hours after a single subcutaneous injection of 6 mg lipegfilgrastim during the first cycle of chemotherapy. After a single subcutaneous injection of 6 mg lipegfilgrastim during the fourth cycle, the maximum blood concentrations were lower than observed in the first cycle (mean values 77 and 111 ng/ml) and were reached after median tmax of 8 hours. The mean terminal half-lives in the fourth cycle were approximately 39 and 42 hours.
In a study (XM22-04) in patients with non-small cell lung cancer receiving chemotherapy consisting of cisplatin and etoposide, the mean maximum blood concentration of 317 ng/ml was reached after a median tmax of 24 hours and the mean terminal half-life was approximately 28 hours after a single subcutaneous injection of 6 mg lipegfilgrastim during the first cycle of chemotherapy. After a single subcutaneous injection of 6 mg lipegfilgrastim during the fourth cycle, the mean maximum blood concentration of 149 ng/ml was reached after a median tmax of 8 hours and the mean terminal half-life was approximately 34 hours.
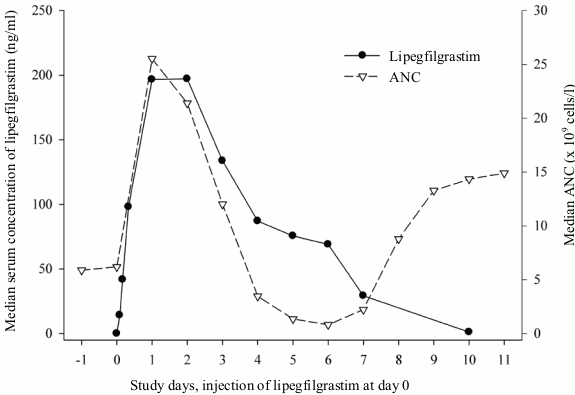
Lipegfilgrastim appears to be mainly eliminated by neutrophil-mediated clearance, which becomes saturated at higher doses. Consistent with a self-regulating clearance mechanism, the serum concentration of lipegfilgrastim declines slowly during the chemotherapy-induced transient neutrophil nadir and rapidly at the following onset of neutrophil recovery (see figure 1).
Figure 1. Profile of median serum concentration of lipegfilgrastim and median ANC in chemotherapy-treated patients after a single 6 mg injection of lipegfilgrastim:
Patients with renal or hepatic impairment
Due to the neutrophil-mediated clearance mechanism, the pharmacokinetics of lipegfilgrastim is not expected to be affected by renal or hepatic impairment.
Elderly patients
Limited patient data indicate that the pharmacokinetics of lipegfilgrastim in elderly patients (65-74 years) is similar to that in younger patients. No pharmacokinetic data are available in patients ≥75 years.
Paediatric population
In a phase 1 study, using a 10 mg/ml solution for subcutaneous injection specifically developed for the paediatric studies, the mean maximum blood concentrations (Cmax) were 243 ng/ml in the 2 to <6-year group, 255 ng/ml in the 6 to <12-year group and 224 ng/ml in the 12 to <18-year group after a single subcutaneous injection of 100 μg/kg (maximum 6 mg) lipegfilgrastim with the first cycle of chemotherapy. The maximum blood concentrations were reached after a median time (tmax) of 23.9 hours, 30.0 hours and 95.8 hours, respectively.
Overweight patients
A trend towards a decrease in lipegfilgrastim exposure was observed with increase in weight. This may result in lowered pharmacodynamic responses in heavy patients (>95 kg). Consequent decrease in efficacy in these patients cannot be excluded on current data.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, single and repeated dose toxicity and local tolerance.
In a study of toxicity to reproduction and development in rabbits, an increased incidence of post-implantation loss and abortion has been observed at high doses of lipegfilgrastim, likely owing to an exaggerated pharmacodynamic effect specific for rabbits. There is no evidence that lipegfilgrastim is teratogenic. These findings are consistent with results from G-CSF and derivatives. Published information on G-CSF and derivatives reveal no evidence of adverse effects on fertility and embryo-foetal development in rats or pre-/postnatal effects other than those related to maternal toxicity as well. There is evidence that filgrastim and pegfilgrastim may be transported at low levels over the placenta in rats, although no information is available for lipegfilgrastim. The relevance of these findings for humans is not known.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.