Loxoprofen
Chemical formula: C₁₅H₁₈O₃ Molecular mass: 246.302 g/mol PubChem compound: 3965
Mechanism of action
Inhibition of prostaglandin biosynthesis constitutes the mechanism of action of loxoprofen, the site of action being cyclo-oxygenase.
When administered orally, loxoprofen sodium hydrate is absorbed from the gastrointestinal tract as an unchanged compound with only a modest gastric-mucosal irritation. It is then rapidly biotransformed into the active metabolite trans-OH form (SRS coordination) with a potent inhibitory effect on prostaglandin biosynthesis to exert its pharmacologic effects.
After being absorbed transdermally, loxoprofen sodium hydrate is biotransformed into an active metabolite trans-OH form (SRS coordination) to exert its excellent anti-inflammatory and analgesic effects in acute inflammations, chronic inflammations and pain.
Pharmacodynamic properties
Loxoprofen sodium hydrate has excellent analgesic, anti-inflammatory and antipyretic properties, with particularly potent pain-relieving activity. This drug is a prodrug which, after being absorbed from the gut, is biotransformed into an active metabolite to exert its actions.
Analgesic effect
Loxoprofen sodium hydrate has been demonstrated to show an ED50 value of 0.13mg/kg in the Randoll-Selitto test (inflamed paw pressuring method: rat, p.o.), the analgesic effect being 10 to 20 times as potent as the reference drugs ketoprofen, naproxen and indomethacin.
Also, the loxoprofen sodium water-based patch has been demonstrated to show the analgesic effect in the Randoll-Selitto test (inflamed paw pressuring method: rat), and in the chronic adjuvant arthritis pain test (rat).
As assessed using the rat thermal inflammatory pain test (rat, p.o.), loxoprofen sodium hydrate showed an ID50 value of 0.76mg/kg and proved to be as potent as naproxen and 3 to >5 times more potent than ketoprofen and indomethacin.
In the chronic arthritis pain test (rat, p.o.), loxoprofen sodium hydrate produced the most profound analgesic effect (ED50: 0.53mg/kg), 4 to 6 times more potent as compared with indomethacin, ketoprofen and naproxen.
The analgesic action of this drug is peripheral.
Anti-inflammatory effect
Loxoprofen sodium hydrate produces an anti-inflammatory effect essentially comparable with the effects of ketoprofen and naproxen on acute and chronic inflammations such as carrageenin-induced edema (rat) and adjuvant arthritis (rat).
Antipyretic effect
Loxoprofen sodium hydrate was demonstrated to exert an antipyretic effect, essentially comparable with the effects of ketoprofen and naproxen and about three times more potent than the effect of indomethacin on yeast-induced fever (rat).
Pharmacokinetic properties
Oral administration
Absorption and Metabolism
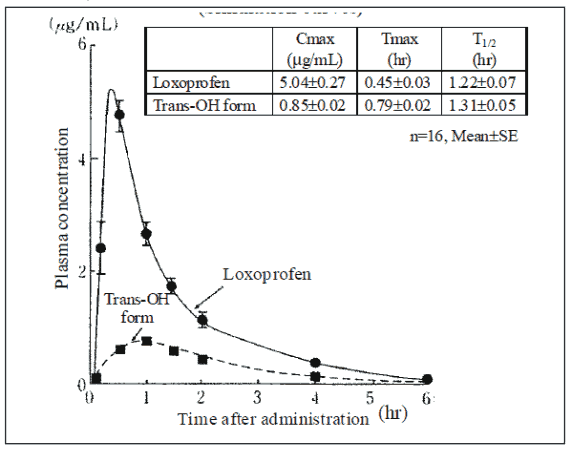
In sixteen healthy adult volunteers, loxoprofen tablets was absorbed rapidly following a single 60-mg oral dose, and loxoprofen (unchanged drug) and its trans-OH form (active metabolite) were demonstrated in blood. The time to peak plasma concentration was about 30 minutes for loxoprofen and about 50 minutes for the trans-OH form, with an approximate half-life of 1 hour and 15 minutes for both compounds.
Plasma concentrations following a single 60-mg dose of loxoprofen tablets (Simulation curves):
Drug-Metabolizing Enzymes
Loxoprofen sodium hydrate did not affect the metabolism of the various drugs that serve as the substrates for cytochrome P450 isoforms (CYP1A1/2, 2A6, 2B6, 2C8/9, 2C19, 2D6, 2E1, and 3A4), even at concentrations approximately 10 times as high as its peak plasma concentration (200 ÌM) in a metabolic inhibition study with human liver microsomes in vitro.
Pharmacokinetics Parameters (single dose)
| Absorption rate constant (hr-1) | Elimination rate constant (hr-1) | |
|---|---|---|
| Loxoprofen | 11.21 ± 1.82 | λ1 = 4.04 ± 0.93 |
| λ2 = 0.59 ± 0.04 | ||
| Trans-OH form | 3.56 ± 0.21 | λ1 = 4.99 ± 0.07 |
| λ2 = 0.54 ± 0.02 |
n=16, Mean±SE
(1) Absorption rate constant and elimination rate constant
(2) Plasma protein binding rate the plasma protein binding rate, as determined in humans (5 subjects at 1 hour after dosing of 60-mg loxoprofen tablets) was 97.0% and 92.8% for loxoprofen and the trans-OH compound, respectively.
(3) AUC8) (n=16, Mean±SE)
Loxoprofen: 6.70 ± 0.26 μg.hr/mL
Trans-OH form: 2.02 ± 0.05 μg.hr/mL
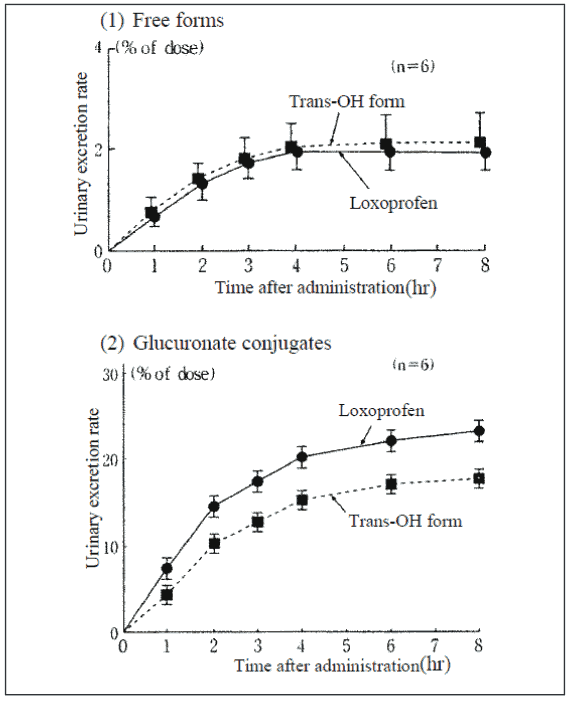
Excretion
Loxoprofen is rapidly excreted in urine; it is excreted largely as glucuronate conjugates of loxoprofen and the trans-OH compound.
Excretion in urine after a single 60-mg dose of loxoprofen tablets:
Excretion in urine over 8 hours after dose (% of dose):
| Free forms | Glucuronate conjugates | |
|---|---|---|
| Loxoprofen | 2.07 ± 0.29 | 21.0 ± 0.4 |
| Trans-OH form | 2.21 ± 0.47 | 16.0 ± 0.6 |
n=6, Mean±SE
Absorption and Excretion Following Multiple Doses
Absorption and excretion of loxoprofen after oral administration at 80mg t.i.d. for 5 days in five healthy adult volunteers did not noticeably differ from those after a single oral dose; hence no evidence of accumulation.
Transdermal administration
Plasma Concentration
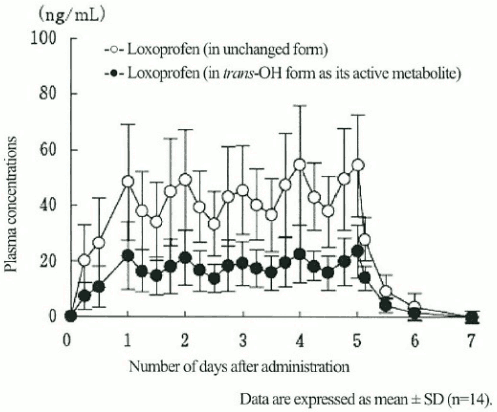
Each of 14 Japanese healthy male adult volunteers was subjected to repeated administration of two Loxoprofen sodium water-based patches on the back once a day for five days, showing detection of loxoprofen and its trans-OH form (active metabolite) in the plasma immediately after the start of the administration with their plasma concentrations gradually increasing over the period of the administration before reaching steady state in four or five days after the administration at low levels compared to those achieved by the equivalent oral dose of the same drug until, upon the discontinuation of the administration, they rapidly disappeared from the plasma to concentrations below the limit of quantitative determination (LQD).
Plasma concentrations of loxoprofen and its trans-OH active metabolite after five-day repitive administration of LOXONIN PAP 100mg (2 patches):
| Css (ng/mL) | AUC0-∞ (ng·hr/mL) | MRT (hr) | |
|---|---|---|---|
| Loxoprofen (in unchanged form) | 54.9 ± 19.3 | 5,281 ± 1,704 | 72.2 ± 4.8 |
| Loxoprofen (in trans-OH form as its active metabolite) | 23.5 ± 9.5 | 2,278 ± 863 | 73.1 ± 4.9 |
Tissue Penetration
Administration of 3.5cm² of the Loxoprofen sodium water-based patch (containing 14C-loxoprofen) to the rat dorsal skin for a time period of 24 hours showed that the concentration of radioactivity in the skeletal muscle immediately under the patch administered area of the skin was 3.6-to 24-fold higher than that in the skeletal muscle under the patch non-administered area of the skin, confirming the formation of the trans-OH form of 14C-loxoprofen as its active metabolite.
Metabolism
An in vitro test of loxoprofen sodium hydrate for metabolism inhibition using human hepatic microsome showed that the drug did not inhibit the metabolism of substrates for cytochrome P450 enzymes (CYP1A1/2, 2A6, 2B6, 2C8/9, 2C19, 2D6, 2E1 and 3A4) even at a concentration of 200µM equivalent to 1,000 times or more than the maximum plasma concentration observed by administration of Loxoprofen sodium water-based patch in a dose of one patch a day.
Urinary Excretion
Each of 14 Japanese healthy male adult volunteers was subjected to repeated administration of two Loxoprofen sodium water-based patches on the back once a day for five days, showing that the daily urinary excretion of loxoprofen, its trans-OH form (active metabolite) and cis-OH form remained almost constant after the elapse of 24 hours following the administration with the total cumulative excretion rate of 2.67% from the start of the administration until 48 hours after the discontinuation of the administration.
Preclinical safety data
Oral administration
No safety information.
Transdermal administration
Toxicity test
Single dose toxicity test
0, 2 and 4% (0, 28.6 and 57.1 mg/body as anhydride respectively) of loxoprofen sodium water-based patch (4 × 5 cm) was percutaneously administered to the trimmed and shaved dorsal skin of Wistar-Imamichi rats (5 males and 5 females per group) for 24 hours (occluded exposure). In each group, no fatal cases were found and no effects were observed on general conditions, body weight and autopsy findings.
Repeated dose toxicity test
Rat:
0, 0.5, 1 and 2% of loxoprofen sodium water-based patch was percutaneously administered to Wistar-Imamichi rats (10 males and 10 females per group) once a day for 3 months. The amount of exposure to loxoprofen sodium hydrate was 0, 10, 20 and 40 mg/kg as anhydride respectively. In the control group, 0, 5, 10 and 20 mg/kg of loxoprofen sodium hydrate as anhydride was orally administered once a day for 3 months. In the male, degeneration in renal papillary interstitium was observed in 1 case among the 1% administered group. In the female, it was observed in 2 cases among both the 0.5% and 1% administered groups and 3 cases among the 2% administered group. The degeneration in kidney was mild, and in the other organs, no apparent toxicity findings were observed even at the highest dose. No-observed-adverse-effect level of loxoprofen sodium water-based patch was estimated to be 0.5% (equivalent to 10 mg/kg) for male and less than 0.5% (equivalent to 10 mg/kg) for female.
Monkey:
0, 1, 2 and 4% of loxoprofen sodium water-based patch was percutaneously administered to cynomolgus monkeys (3 males and 3 females per group) once a day (21 to 26 hours) for 3 months (13 weeks) (occluded exposure). No changes were observed in general conditions, body weight, food consumption, and haematology, blood chemical and pathology tests. No-observed-adverse-effect level of loxoprofen sodium water-based patch was estimated to be equal to or higher than 4% in both male and female.
Other specific toxicity
Local irritation, skin sensitization, skin photosensitization and phototoxicity tests were performed using the base of loxoprofen sodium water-based patch and 1, 2 and 4% of loxoprofen sodium water-based patch. No toxicity findings were observed.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.