Pamidronate Disodium Other names: Pamidronic acid
Chemical formula: C₃H₁₁NO₇P₂ Molecular mass: 235.07 g/mol PubChem compound: 4674
Mechanism of action
The principal pharmacologic action of pamidronic acid is inhibition of bone resorption. Although the mechanism of antiresorptive action is not completely understood, several factors are thought to contribute to this action. Pamidronic acid adsorbs to calcium phosphate (hydroxyapatite) crystals in bone and may directly block dissolution of this mineral component of bone.
In vitro studies also suggest that inhibition of osteoclast activity contributes to inhibition of bone resorption. In animal studies, at doses recommended for the treatment of hypercalcemia, pamidronic acid inhibits bone resorption apparently without inhibiting bone formation and mineralization. Of relevance to the treatment of hypercalcemia of malignancy is the finding that pamidronic acid inhibits the accelerated bone resorption that results from osteoclast hyperactivity induced by various tumors in animal studies.
Pharmacodynamic properties
Serum phosphate levels have been noted to decrease after administration of pamidronic acid, presumably because of decreased release of phosphate from bone and increased renal excretion as parathyroid hormone levels, which are usually suppressed in hypercalcemia associated with malignancy, return toward normal. Phosphate therapy was administered in 30% of the patients in response to a decrease in serum phosphate levels. Phosphate levels usually returned toward normal within 7-10 days.
Urinary calcium/creatinine and urinary hydroxyproline/creatinine ratios decrease and usually return to within or below normal after treatment with pamidronic acid. These changes occur within the first week after treatment, as do decreases in serum calcium levels, and are consistent with an antiresorptive pharmacologic action.
Pharmacokinetic properties
Pharmacokinetics
Cancer patients (n=24) who had minimal or no bony involvement were given an intravenous infusion of 30, 60, or 90 mg of pamidronic acid over 4 hours and 90 mg of pamidronic acid over 24 hours (Table 1).
Distribution
The mean ± SD body retention of pamidronate was calculated to be 54 ± 16% of the dose over 120 hours.
Metabolism
Pamidronate is not metabolized and is exclusively eliminated by renal excretion.
Excretion
After administration of 30, 60, and 90 mg of pamidronic acid over 4 hours, and 90 mg of pamidronic acid over 24 hours, an overall mean ± SD of 46 ± 16% of the drug was excreted unchanged in the urine within 120 hours. Cumulative urinary excretion was linearly related to dose. The mean ± SD elimination half-life is 28 ± 7 hours. Mean ± SD total and renal clearances of pamidronate were 107 ± 50 mL/min and 49 ± 28 mL/min, respectively. The rate of elimination from bone has not been determined.
Special Populations
There are no data available on the effects of age, gender, or race on the pharmacokinetics of pamidronate.
Pediatric
Pamidronate is not labeled for use in the pediatric population.
Renal Insufficiency
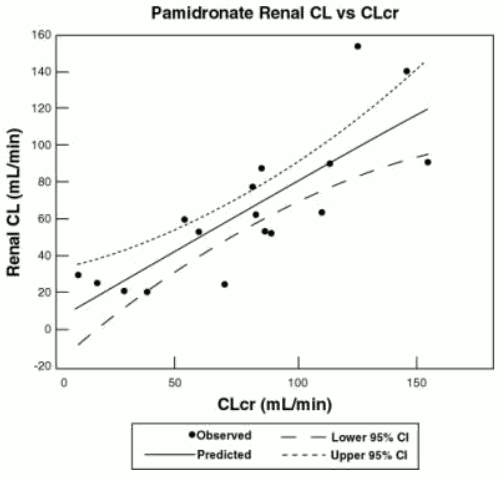
The pharmacokinetics of pamidronate were studied in cancer patients (n=19) with normal and varying degrees of renal impairment. Each patient received a single 90-mg dose of pamidronic acid infused over 4 hours. The renal clearance of pamidronate in patients was found to closely correlate with creatinine clearance (see Figure 1). A trend toward a lower percentage of drug excreted unchanged in urine was observed in renally impaired patients. Adverse experiences noted were not found to be related to changes in renal clearance of pamidronate. Given the recommended dose, 90 mg infused over 4 hours, excessive accumulation of pamidronate in renally impaired patients is not anticipated if pamidronic acid is administered on a monthly basis.
Figure 1. Pamidronate renal clearance as a function of creatinine clearance in patients with normal and impaired renal function. The lines are the mean prediction line and 95% confidence intervals:
Hepatic Insufficiency
The pharmacokinetics of pamidronate were studied in male cancer patients at risk for bone metastases with normal hepatic function (n=6) and mild to moderate hepatic dysfunction (n=7). Each patient received a single 90-mg dose of pamidronic acid infused over 4 hours. Although there was a statistically significant difference in the pharmacokinetics between patients with normal and impaired hepatic function, the difference was not considered clinically relevant. Patients with hepatic impairment exhibited higher mean AUC (53%) and Cmax (29%), and decreased plasma clearance (33%) values. Nevertheless, pamidronate was still rapidly cleared from the plasma. Drug levels were not detectable in patients by 12 to 36 hours after drug infusion. Because pamidronic acid is administered on a monthly basis, drug accumulation is not expected. No changes in pamidronic acid dosing regimen are recommended for patients with mild to moderate abnormal hepatic function. Pamidronic acid has not been studied in patients with severe hepatic impairment.
Drug-Drug Interactions
There are no human pharmacokinetic data for drug interactions with pamidronic acid.
Table 1. Mean (SD, CV%) Pamidronate Pharmacokinetic Parameters in Cancer Patients(n=6 for each group):
| Dose (infusion rate) | Maximum Concentration (μg/mL) | Percent of dose excreted in urine | Total Clearance (mL/min) | Renal Clearance (mL/min) |
|---|---|---|---|---|
| 30 mg (4 hrs) | 0.73 (0.14, 19.1%) | 43.9 (14.0, 31.9%) | 136 (44, 32.4%) | 58 (27, 46.5%) |
| 60 mg (4 hrs) | 1.44 (0.57, 39.6%) | 47.4 (47.4, 54.4%) | 88 (56, 63.6%) | 42 (28, 66.7%) |
| 90 mg (4 hrs) | 2.61 (0.74, 28.3%) | 45.3 (25.8, 56.9%) | 103 (37, 35.9%) | 44 (16, 36.4%) |
| 90 mg (24 hrs) | 1.38 (1.97, 142.7%) | 47.5 (10.2, 21.5%) | 101 (58, 57.4%) | 52 (42, 80.8%) |
After intravenous administration of radiolabeled pamidronate in rats, approximately 50%-60% of the compound was rapidly adsorbed by bone and slowly eliminated from the body by the kidneys. In rats given 10 mg/kg bolus injections of radiolabeled pamidronic acid, approximately 30% of the compound was found in the liver shortly after administration and was then redistributed to bone or eliminated by the kidneys over 24-48 hours. Studies in rats injected with radiolabeled pamidronic acid showed that the compound was rapidly cleared from the circulation and taken up mainly by bones, liver, spleen, teeth, and tracheal cartilage. Radioactivity was eliminated from most soft tissues within 1-4 days; was detectable in liver and spleen for 1 and 3 months, respectively; and remained high in bones, trachea, and teeth for 6 months after dosing. Bone uptake occurred preferentially in areas of high bone turnover. The terminal phase of elimination half-life in bone was estimated to be approximately 300 days.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.