Sodium tetradecyl sulfate
Chemical formula: C₁₄H₂₉NaO₄S Molecular mass: 316.43 g/mol PubChem compound: 23665772
Pregnancy
Safety for use in pregnancy has not been established. There are no or limited amount of data from the use of sodium tetradecyl sulfate in pregnant women. Animal studies are insufficient with respect to reproductive toxicity. Treatment should be postponed until after childbirth.
Sodium tetradecyl sulfate should be used only when clearly needed for symptomatic relief and when the potential benefits outweigh the potential hazards to the fetus.
Nursing mothers
It is not known whether sodium tetradecyl sulfate is excreted in human milk. Caution should be exercised when used in nursing mothers.
Carcinogenesis, mutagenesis and fertility
Fertility
It is not known whether sodium tetradecyl sulfate affects fertility.
Effects on ability to drive and use machines
Sodium tetradecyl sulfate has no or negligible direct influence on the ability to drive and use machines. However, a bandage and/or compression stockings may be added after treatment. This could affect the ability to drive.
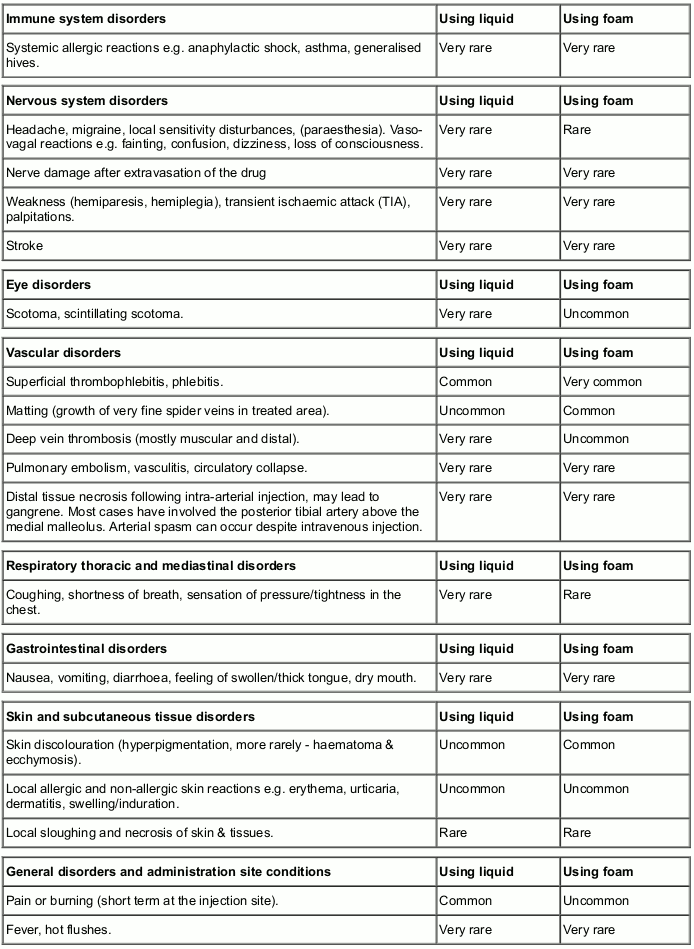
Adverse reactions
The most commonly reported side effects are pain on injection urticaria, superficial thrombophlebitis and temporary skin pigmentation after treatment. Very rarely a permanent discoloration may remain along the path of the sclerosed vein segment. Ulceration may occur following extravasation of the drug. It is important to use the lowest strength that will sclerose the vein as many of the common side effects are caused by using a concentration that is too high.
Intra-arterial injection although very rare has been reported resulting in significant tissue necrosis including loss of the extremity.
The most serious side effects are anaphylactic shock and pulmonary embolism and deaths have been reported in patients receiving sodium tetradecyl sulfate.
Adverse events are listed below by system organ class and estimated frequency from published clinical data. Frequencies are defined using the following convention:
Very common: ≥1/10
Common: ≥1/100 to <1/10
Uncommon: ≥1/1,000 to <1/100
Rare: ≥1/10,000 to <1/1,000
Very rare: (includes isolated reports) <1/10,000
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.