Valrubicin
Chemical formula: C₃₄H₃₆F₃NO₁₃ Molecular mass: 723.651 g/mol PubChem compound: 454216
Pharmacodynamic properties
Valrubicin is an anthracycline that affects a variety of inter-related biological functions, most of which involve nucleic acid metabolism. It readily penetrates into cells, where it inhibits the incorporation of nucleosides into nucleic acids, causes extensive chromosomal damage, and arrests cell cycle in G2. Although valrubicin does not bind strongly to DNA, a principal mechanism of its action, mediated by valrubicin metabolites, is interference with the normal DNA breaking-resealing action of DNA topoisomerase II.
Pharmacokinetic properties
Pharmacokinetics after Intravesical Administration of Valrubicin
When 800 mg valrubicin was administered intravesically to patients with carcinoma in situ, valrubicin penetrated into the bladder wall. The mean total anthracycline concentration measured in bladder tissue exceeded the levels causing 90% cytotoxicity to human bladder cells cultured in vitro. During the two-hour dose-retention period, the metabolism of valrubicin to its major metabolites N-trifluoroacetyladriamycin and N-trifluoroacetyladriamycinol was negligible. After retention, the drug was almost completely excreted by voiding the instillate. Mean percent recovery of valrubicin, N-trifluoroacetyladriamycin, and total anthracyclines in 14 urine samples from six patients was 98.6%, 0.4%, and 99.0% of the total administered drug, respectively. During the two-hour dose-retention period, only nanogram quantities of valrubicin were absorbed into the plasma. Valrubicin metabolites N-trifluoroacetyladriamycin and Ntrifluoroacetyladriamycinol were measured in blood.
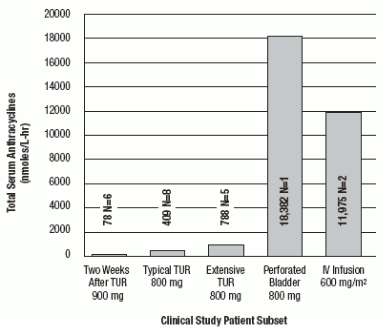
Total systemic exposure to anthracyclines during and after intravesical administration of valrubicin is dependent upon the condition of the bladder wall. The mean AUC0-6hours (total anthracyclines exposure) for an intravesical dose of 900 mg of valrubicin administered 2 weeks after transurethral resection of bladder tumors (n=6) was 78 nmol/L•hr. In patients receiving 800 mg of valrubicin 5 to 51 minutes after typical (n=8) and extensive (n=5) transurethral resection of bladder tumors (TURBs), the mean AUC0-6hours values for total anthracyclines were 409 and 788 nmol/L•hr, respectively. The AUC0-6hours total exposure to anthracyclines was 18,382 nmol/L•hr in one patient who experienced a perforated bladder following a transurethral resection that occurred 5 minutes before administration of an intravesical dose of 800 mg of valrubicin. Administration of a comparable intravenous dose of valrubicin (600 mg/m²; n=2) as a 24-hour infusion resulted in an AUC0-6hours for total anthracyclines of 11,975 nmol/L•hr. These results are shown in FIGURE 2.
FIGURE 1. Comparison of Mean AUC0-6hours in valrubicin Clinical Studies (N=number of patients):
The patient with a perforated bladder who received 800 mg of valrubicin intravesically developed severe leukopenia and neutropenia approximately two weeks after drug administration. Systemic hematologic toxicity from valrubicin was not seen after an intravesical dose of 800 mg of valrubicin unless perforation of the urinary bladder occurred.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.