SPRAVATO Nasal spray, solution Ref.[10286] Active ingredients: Esketamine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
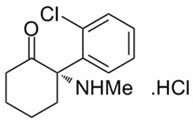
SPRAVATO contains esketamine hydrochloride, a non-competitive NmethylD-aspartate (NMDA) receptor antagonist. Esketamine is the S-enantiomer of racemic ketamine. The chemical name is (S)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride. Its molecular formula is C13H16ClNO.HCl and its molecular weight is 274.2.
The structural formula is:
Esketamine hydrochloride is a white or almost white crystalline powder that is freely soluble in water and in methanol, and soluble in ethanol.
SPRAVATO nasal spray is intended for nasal administration. Esketamine hydrochloride is contained as a solution in a stoppered glass vial within the nasal spray device. Each device delivers two sprays with a total of 32.3 mg of esketamine hydrochloride (equivalent to 28 mg of esketamine) in 0.2 mL of a clear, colorless aqueous solution with a pH of 4.5.
The inactive ingredients are citric acid monohydrate, edetate disodium, sodium hydroxide, and water for injection.
| Dosage Forms and Strengths |
|---|
|
Nasal Spray: 28 mg of esketamine per device. Each nasal spray device delivers two sprays containing a total of 28 mg esketamine. |
| How Supplied |
|---|
|
SPRAVATO nasal spray is available as an aqueous solution of esketamine hydrochloride in a stoppered glass vial within a nasal spray device. Each nasal spray device delivers two sprays containing a total of 28 mg of esketamine (supplied as 32.3 mg of esketamine hydrochloride). SPRAVATO is available in the following presentations:
Within each kit, each 28 mg device is individually packaged in a sealed blister (NDC 50458-028-00). |
Drugs
| Drug | Countries | |
|---|---|---|
| SPRAVATO | Austria, Brazil, Canada, Cyprus, Ecuador, Estonia, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Lithuania, Netherlands, New Zealand, Poland, Romania, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.