ELOCON Cream, 0.1% Ref.[10556] Active ingredients: Mometasone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
ELOCON (mometasone furoate) Cream, 0.1% contains mometasone furoate for topical use. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity.
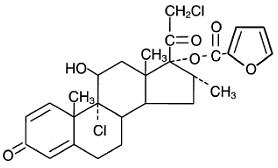
Chemically, mometasone furoate is 9α,21-dichloro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C27H30Cl2O6, a molecular weight of 521.4 and the following structural formula:
Mometasone furoate is a white to off-white powder practically insoluble in water, slightly soluble in octanol, and moderately soluble in ethyl alcohol.
Each gram of ELOCON Cream, 0.1% contains 1 mg mometasone furoate in a white to off-white cream base of aluminum starch octenylsuccinate (Gamma Irradiated), hexylene glycol, hydrogenated soybean lecithin, phosphoric acid, purified water, titanium dioxide, white soft paraffin, and white wax.
| Dosage Forms and Strengths |
|---|
|
Cream, 0.1%. Each gram of ELOCON Cream contains 1 mg of mometasone furoate in a white to off-white smooth and homogenous cream base. |
| How Supplied |
|---|
|
ELOCON Cream is white to off-white in color and supplied in 15-gram (NDC 0085-3149-01) and 50-gram (NDC 0085-3149-03) tubes. Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA Manufactured by: Schering-Plough Labo NV, Heist-op-den-Berg, Belgium |
Drugs
| Drug | Countries | |
|---|---|---|
| ELOCON | Austria, Australia, Cyprus, Germany, Estonia, Finland, Ireland, Lithuania, Netherlands, New Zealand, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.