IMITREX Nasal spray Ref.[10574] Active ingredients: Sumatriptan
Source: FDA, National Drug Code (US) Revision Year: 2017
Product description
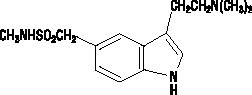
IMITREX nasal spray contains sumatriptan, a selective 5-HT1B/1D receptor agonist. Sumatriptan is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide, and it has the following structure:
The empirical formula is C14H21N3O2S, representing a molecular weight of 295.4. Sumatriptan is a white to off-white powder that is readily soluble in water and in saline.
Each IMITREX nasal spray contains 5 or 20 mg of sumatriptan in a 100-microL unit dose aqueous buffered solution containing monobasic potassium phosphate NF, anhydrous dibasic sodium phosphate USP, sulfuric acid NF, sodium hydroxide NF, and purified water USP. The pH of the solution is approximately 5.5. The osmolality of the solution is 372 or 742 mOsmol for the 5- and 20-mg IMITREX nasal spray, respectively.
| Dosage Forms and Strengths |
|---|
|
Unit dose nasal spray devices containing 5 mg or 20 mg sumatriptan. |
| How Supplied |
|---|
|
IMITREX nasal spray 5 mg (NDC 0173-0524-00) and 20 mg (NDC 0173-0523-00) are each supplied in boxes of 6 nasal spray devices. Each unit dose spray supplies 5 mg and 20 mg, respectively, of sumatriptan. GlaxoSmithKline, Research Triangle Park, NC 27709 |
Drugs
| Drug | Countries | |
|---|---|---|
| IMITREX | Canada, Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.