IMITREX Nasal spray Ref.[10574] Active ingredients: Sumatriptan
Source: FDA, National Drug Code (US) Revision Year: 2017
12.1. Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache through agonist effects at the 5‑HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
12.2. Pharmacodynamics
Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis, has been reported in patients with and without a history of hypertension [see Warnings and Precautions (5.8)].
Peripheral (Small) Arteries
In healthy volunteers (N=18), a trial evaluating the effects of sumatriptan on peripheral (small vessel) arterial reactivity failed to detect a clinically significant increase in peripheral resistance.
Heart Rate
Transient increases in blood pressure observed in some patients in clinical trials carried out during sumatriptan’s development as a treatment for migraine were not accompanied by any clinically significant changes in heart rate.
12.3. Pharmacokinetics
Absorption
In a trial of 20 female volunteers, the mean maximum concentration following a 5- and 20-mg intranasal dose was 5 and 16 ng/mL, respectively. The mean Cmax following a 6‑mg subcutaneous injection is 71 ng/mL (range: 49 to 110 ng/mL). The mean Cmax is 18 ng/mL (range: 7 to 47 ng/mL) following oral dosing with 25 mg and 51 ng/mL (range: 28 to 100 ng/mL) following oral dosing with 100 mg of sumatriptan. In a trial of 24 male volunteers, the bioavailability relative to subcutaneous injection was low, approximately 17%, primarily due to presystemic metabolism and partly due to incomplete absorption.
Clinical and pharmacokinetic data indicate that administration of two 5-mg doses, 1 dose in each nostril, is equivalent to administration of a single 10-mg dose in 1 nostril.
Distribution
Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL, is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The apparent volume of distribution is 2.7 L/kg.
Metabolism
In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
Elimination
The elimination half-life of sumatriptan administered as a nasal spray is approximately 2 hours, similar to the half-life seen after subcutaneous injection. Only 3% of the dose is excreted in the urine as unchanged sumatriptan; 42% of the dose is excreted as the major metabolite, the indole acetic acid analogue of sumatriptan. The total plasma clearance is approximately 1,200 mL/min.
Specific Populations
Age
The pharmacokinetics of sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in subjects with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years). Intranasal sumatriptan has not been evaluated for age differences.
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of sumatriptan has not been examined.
Patients with Hepatic Impairment
The effect of mild to moderate hepatic disease on the pharmacokinetics of the intranasal formulation of sumatriptan has not been evaluated. Sumatriptan bioavailability following intranasal administration is 17%, similar to that after oral administration (15%). Following oral administration, an approximately 70% increase in Cmax and AUC was observed in one small trial of patients with moderate liver impairment (n=8) matched for sex, age, and weight with healthy subjects (n=8). Similar changes can be expected following intranasal administration.
The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied. The use of IMITREX nasal spray in patients with severe hepatic impairment is contraindicated [see Contraindications (4)].
Racial Groups
The systemic clearance and Cmax of subcutaneous sumatriptan were similar in black (n=34) and Caucasian (n=38) healthy male subjects. Intranasal sumatriptan has not been evaluated for race differences.
Drug Interaction Studies
Monoamine Oxidase-A Inhibitors
Treatment with MAO-A inhibitors generally leads to an increase of sumatriptan plasma levels [see Contraindications (4), Drug Interactions (7.2)]. MAO inhibitors interaction studies have not been performed with intranasal sumatriptan.
Due to gut and hepatic metabolic first-pass effects, the increase of systemic exposure after coadministration of an MAO-A inhibitor with oral sumatriptan is greater than after coadministration of the MAO inhibitors with subcutaneous sumatriptan. The effects of an MAO inhibitor on systemic exposure after intranasal sumatriptan would be expected to be greater than the effect after subcutaneous sumatriptan but smaller than the effect after oral sumatriptan because only swallowed drug would be subject to first-pass effects.
In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half‑life.
A small trial evaluating the effect of pretreatment with an MAO-A inhibitor on the bioavailability from a 25-mg oral sumatriptan tablet resulted in an approximately 7-fold increase in systemic exposure.
Xylometazoline
An in vivo drug interaction trial indicated that 3 drops of xylometazoline (0.1% w/v), a decongestant, administered 15 minutes prior to a 20-mg nasal dose of sumatriptan did not alter the pharmacokinetics of sumatriptan.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In carcinogenicity studies in mouse and rat in which sumatriptan was administered orally for 78 and 104 weeks, respectively, there was no evidence in either species of an increase in tumors related to sumatriptan administration.
Carcinogenicity studies of sumatriptan using the nasal route have not been conducted.
Mutagenesis
Sumatriptan was negative in in vitro (bacterial reverse mutation [Ames], gene cell mutation in Chinese hamster V79/HGPRT, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility
When sumatriptan (5, 50, or 500 mg/kg/day) was administered orally to male and female rats prior to and throughout the mating period, there was a treatment-related decrease in fertility secondary to a decrease in mating in animals treated with doses greater than 5 mg/kg/day. It is not clear whether this finding was due to an effect on males or females or both.
When sumatriptan was administered by subcutaneous injection to male and female rats prior to and throughout the mating period, there was no evidence of impaired fertility at doses up to 60 mg/kg/day.
Fertility studies of sumatriptan using the intranasal route have not been conducted.
13.2. Animal Toxicology and/or Pharmacology
Corneal Opacities
Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dose tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60‑week study. Earlier examinations for these toxicities were not conducted and no-effect doses were not established.
14. Clinical Studies
The efficacy of IMITREX nasal spray in the acute treatment of migraine headaches was demonstrated in 8 randomized, double-blind, placebo-controlled trials, of which 5 used the recommended dosing regimen and used the marketed formulation. Patients enrolled in these 5 trials were predominately female (86%) and Caucasian (95%), with a mean age of 41 years (range: 18 to 65 years). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed up to 2 hours after dosing. Associated symptoms such as nausea, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours postdose. A second dose of IMITREX nasal spray or other medication was allowed 2 to 24 hours after the initial treatment for recurrent headache. The frequency and time to use of these additional treatments were also determined. In all trials, doses of 10 and 20 mg were compared with placebo in the treatment of 1 to 3 migraine attacks. Patients received doses as a single spray into 1 nostril. In 2 trials, a 5-mg dose was also evaluated.
In all 5 trials utilizing the market formulation and recommended dosage regimen, the percentage of patients achieving headache response 2 hours after treatment was significantly greater among patients receiving IMITREX nasal spray at all doses (with one exception) compared with those who received placebo. In 4 of the 5 trials, there was a statistically significant greater percentage of patients with headache response at 2 hours in the 20-mg group when compared with the lower-dose groups (5 and 10 mg). There were no statistically significant differences between the 5- and 10-mg dose groups in any trial. The results from the 5 controlled clinical trials are summarized in Table 2. Note that, in general, comparisons of results obtained in trials conducted under different conditions by different investigators with different samples of patients are ordinarily unreliable for purposes of quantitative comparison.
Table 2. Percentage of Patients with Headache Response (No or Mild Pain) 2 Hours following Treatment:
| IMITREX Nasal Spray 5 mg | IMITREX Nasal Spray 10 mg | IMITREX Nasal Spray 20 mg | Placebo | |
|---|---|---|---|---|
| Trial 1 | 49%a | 46%a | 64%a,b,c | 25% |
| (n=121) | (n=112) | (n=118) | (n=63) | |
| Trial 2 | Not applicable | 44%a | 55%a,b | 25% |
| (n=273) | (n=277) | (n=138) | ||
| Trial 3 | Not applicable | 54%a | 63%a | 35% |
| (n=106) | (n=202) | (n=100) | ||
| Trial 4 | Not applicable | 43% | 62% a,b | 29% |
| (n=106) | (n=215) | (n=112) | ||
| Trial 5d | 45%a | 53%a | 60%a,c | 36% |
| (n=296) | (n=291) | (n=286) | (n=198) |
a P<0.05 in comparison with placebo.
b P<0.05 in comparison with 10 mg.
c P<0.05 in comparison with 5 mg.
d Data are for attack 1 only of multi-attack trial for comparison.
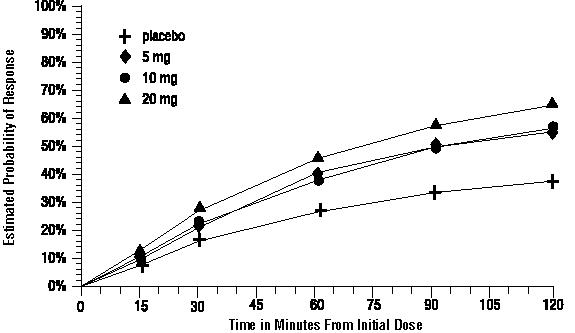
The estimated probability of achieving an initial headache response over the 2 hours following treatment is depicted in Figure 1.
Figure 1. Estimated Probability of Achieving Initial Headache Response within 120 Minutesa:
a The figure shows the probability over time of obtaining headache response (no or mild pain) following treatment with intranasal sumatriptan. The averages displayed are based on pooled data from the 5 clinical controlled trials providing evidence of efficacy. Kaplan-Meier plot with patients not achieving response within 120 minutes censored to 120 minutes.
For patients with migraine-associated nausea, photophobia, and phonophobia at baseline, there was a lower incidence of these symptoms at 2 hours following administration of IMITREX nasal spray compared with placebo.
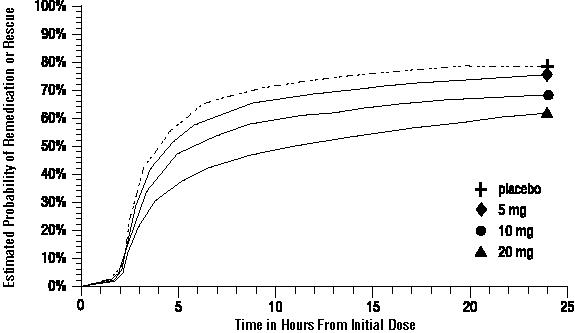
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure 2. The Estimated Probability of Patients Taking a Second Dose or Other Medication for Migraine over the 24 Hours following the Initial Dose of Study Treatmenta:
a Kaplan-Meier plot based on data obtained in the 3 clinical controlled trials providing evidence of efficacy with patients not using additional treatments censored to 24 hours. Plot also includes patients who had no response to the initial dose. No remedication was allowed within 2 hours postdose.
There is evidence that doses above 20 mg do not provide a greater effect than 20 mg. There was no evidence to suggest that treatment with sumatriptan was associated with an increase in the severity of recurrent headaches. The efficacy of IMITREX nasal spray was unaffected by presence of aura; duration of headache prior to treatment; gender, age, or weight of the subject; or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants). There were insufficient data to assess the impact of race on efficacy.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.