IMITREX Solution for injection Ref.[10575] Active ingredients: Sumatriptan
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
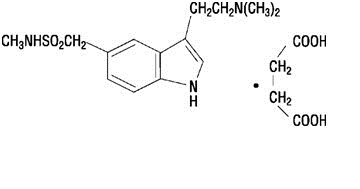
IMITREX injection contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
IMITREX injection is a clear, colorless to pale yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 0.5 mL of IMITREX injection 8-mg/mL solution contains 5.6 mg of sumatriptan succinate equivalent to 4 mg of sumatriptan and 3.8 mg of sodium chloride, USP in Water for Injection, USP. Each 0.5 mL of IMITREX injection 12-mg/mL solution contains 8.4 mg of sumatriptan succinate equivalent to 6 mg of sumatriptan and 3.5 mg of sodium chloride, USP in Water for Injection, USP. The pH range of both solutions is approximately 4.2 to 5.3. The osmolality of both injections is 291 mOsmol.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
IMITREX injection contains sumatriptan (base) as the succinate salt and is supplied as a clear, colorless to pale yellow, sterile, nonpyrogenic solution as follows: Prefilled Syringe and/or Autoinjector Pen: The needle shield of the prefilled syringe contains dry natural rubber (a latex derivative) that has the potential to cause allergic reactions in latex-sensitive individuals. Each pack contains a Patient Information and Instructions for Use leaflet.
Single-Dose Vial:
GlaxoSmithKline, Research Triangle Park, NC 27709 |
Drugs
| Drug | Countries | |
|---|---|---|
| IMITREX | Canada, Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.