LANOXIN Solution for injection Ref.[10580] Active ingredients: Digoxin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
LANOXIN (digoxin) is one of the cardiac (or digitalis) glycosides, a closely related group of drugs having in common specific effects on the myocardium. These drugs are found in a number of plants. Digoxin is extracted from the leaves of Digitalis lanata. The term “digitalis” is used to designate the whole group of glycosides. The glycosides are composed of 2 portions: a sugar and a cardenolide (hence “glycosides”).
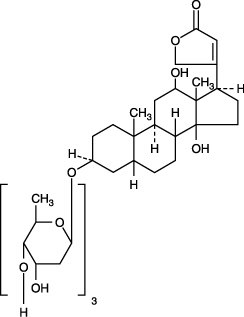
Digoxin is described chemically as (3β,5β,12β)3[(O-2,6-dideoxy-β-D-ribohexopyranosyl(1→4)-O-2,6-dideoxy-β-D-ribohexopyranosyl(1→4)-2,6-dideoxy-β-D-ribo-hexopyranosyl)oxy]-12,14-dihydroxy-card-20(22)-enolide.
Its molecular formula is C41H64O14, its molecular weight is 780.95, and its structural formula is:
Digoxin exists as odorless white crystals that melt with decomposition above 230°C. The drug is practically insoluble in water and in ether; slightly soluble in diluted (50%) alcohol and in chloroform; and freely soluble in pyridine.
LANOXIN Injection and Injection Pediatric are sterile solutions of digoxin for intravenous or intramuscular injection. The vehicle contains 42.5% (W/V) propylene glycol and 10% alcohol (V/V). The injection is buffered to a pH of 6.8-7.2 with 0.17% dibasic sodium phosphate and 0.08% anhydrous citric acid. Each 2-mL ampule or vial of LANOXIN Injection contains 500 mcg (0.5 mg) digoxin (250 mcg [0.25 mg] per mL). Dilution is not required. Each 1-mL ampule or vial of LANOXIN Injection Pediatric contains 100 mcg (0.1 mg) digoxin. Dilution is not required.
| Dosage Forms and Strengths |
|---|
|
LANOXIN Injection: Ampules of 500 mcg (0.5 mg) in 2 mL (250 mcg [0.25 mg] per 1 mL). LANOXIN Injection Pediatric: Ampules of 100 mcg (0.1 mg) in 1 mL. |
| How Supplied |
|---|
|
LANOXIN (digoxin) Injection, 500 mcg (0.5 mg) in 2 mL (250 mcg [0.25 mg] per mL); box of 10 ampules (NDC 70515 260 10) LANOXIN (digoxin) Injection, 500 mcg (0.5 mg) in 2 mL (250 mcg [0.25 mg] per mL); box of 10 vials (NDC 70515 261 10) LANOXIN (digoxin) Injection Pediatric, 100 mcg (0.1 mg) in 1 mL; box of 10 ampules (NDC 70515 262 10) LANOXIN (digoxin) Injection Pediatric, 100 mcg (0.1 mg) in 1 mL; box of 10 vials (NDC 70515 263 10) LANOXIN is registered trademark of GlaxoSmithKline. Manufactured for Covis Pharma, Zug, 6300 Switzerland |
Drugs
| Drug | Countries | |
|---|---|---|
| LANOXIN | Australia, Cyprus, Hong Kong, Ireland, Israel, Lithuania, Malta, Mexico, Netherlands, New Zealand, Singapore, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.