PHENERGAN Solution for injection Ref.[10597] Active ingredients: Promethazine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
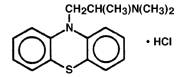
PHENERGAN Injection (promethazine hydrochloride injection, USP), is a sterile, pyrogen-free solution for deep intramuscular or intravenous administration. Promethazine hydrochloride (10 HPhenothiazine-10-ethanamine, N,N,α-trimethyl, monohydrochloride, (±)-) is a racemic compound and has the following structural formula:
C17H21ClN2S
MW 320.88
Each mL contains promethazine hydrochloride, either 25 mg or 50 mg, edetate disodium 0.1 mg, calcium chloride 0.04 mg, sodium metabisulfite 0.25 mg and phenol 5 mg in Water for Injection. pH 4.0 to 5.5; buffered with acetic acid-sodium acetate.
PHENERGAN Injection (promethazine hydrochloride injection, USP) is a clear, colorless solution. The product is light sensitive. It should be inspected before use and discarded if either color or particulate is observed.
| How Supplied |
|---|
|
PHENERGAN Injection (promethazine hydrochloride injection, USP) is available as follows: 25 mg/Ml NDC: 70518-2656-00 PACKAGING: 25 in 1 CARTON NDC: 70518-2656-01 PACKAGING: 1 mL in 1 VIAL TYPE 0 Repackaged and Distributed By: Remedy Repack, Inc., 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762 |
Drugs
| Drug | Countries | |
|---|---|---|
| PHENERGAN | France, Ireland, Israel, Malta, New Zealand, Tunisia, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.