XENICAL Capsule Ref.[10625] Active ingredients: Orlistat
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
XENICAL (orlistat) is a gastrointestinal lipase inhibitor for obesity management that acts by inhibiting the absorption of dietary fats.
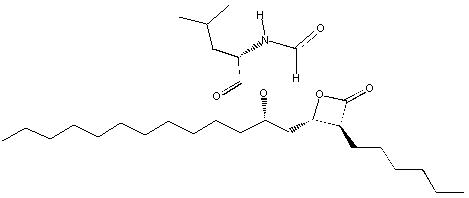
Orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)1[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its empirical formula is C29H53NO5, and its molecular weight is 495.7. It is a single diastereomeric molecule that contains four chiral centers, with a negative optical rotation in ethanol at 529 nm.
The structure is:
Orlistat is a white to off-white crystalline powder. Orlistat is practically insoluble in water, freely soluble in chloroform, and very soluble in methanol and ethanol. Orlistat has no p Ka within the physiological pH range.
XENICAL is available for oral administration as a turquoise hard-gelatin capsule. The capsule is imprinted with black. Each capsule contains a pellet formulation consisting of 120 mg of the active ingredient, orlistat, as well as the inactive ingredients microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, povidone, and talc. The capsule shell contains gelatin, titanium dioxide, and FD&C Blue No. 2 with black printing ink containing pharmaceutical grade shellac, propylene glycol, strong ammonium solution, potassium hydroxide and black iron oxide.
| Dosage Forms and Strengths |
|---|
|
XENICAL 120 mg turquoise capsules imprinted with XENICAL 120 in black ink. |
| How Supplied |
|---|
|
XENICAL is a turquoise, hard-gelatin capsule containing pellets of powder. XENICAL 120 mg Capsules: Turquoise, two-piece, No. 1 opaque hard-gelatin capsule imprinted with XENICAL 120 in black ink — bottle of 90 (NDC 61269-460-90). XENICAL is a registered trademark of CHEPLAPHARM Arzneimittel GmbH. Licensed by: CHEPLAPHARM Arzneimittel GmbH, Ziegelhof 24, 17489 Greifswald, Germany Distributed by: H2-Pharma, LLC, 2010 Berry Chase Place, Montgomery, AL 36117, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| XENICAL | Austria, Australia, Canada, Cyprus, Ecuador, Estonia, Spain, France, Hong Kong, Croatia, Ireland, Israel, Italy, Lithuania, Netherlands, New Zealand, Poland, Romania, Singapore, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.