XENICAL Capsule Ref.[10625] Active ingredients: Orlistat
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Orlistat is a reversible inhibitor of gastrointestinal lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipases. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control.
12.2. Pharmacodynamics
Dose-response Relationship
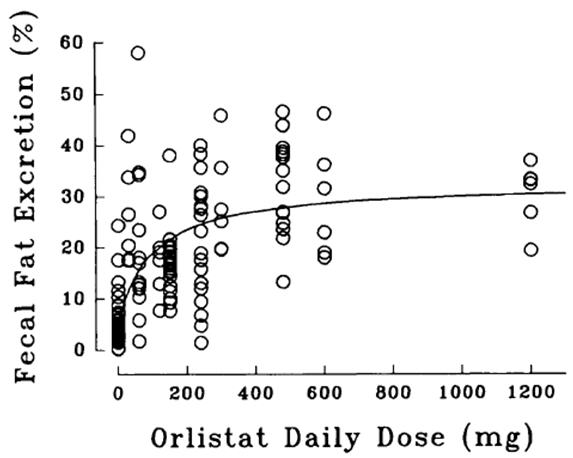
The dose-response relationship for orlistat in human volunteers is shown in Figure 1. The effect is the percentage of ingested fat excreted, referred to as fecal fat excretion percentage. Both individual data (open circles) and the curve predicted for the population with the maximum-effect model (continuous line) are shown in Figure 1.
Figure 1. Dose-Response Relationship for Orlistat in Human Volunteers:
At the recommended therapeutic dose of 120 mg three times a day, orlistat inhibits dietary fat absorption by approximately 30%.
Ethanol does not affect orlistat’s effect on preventing the absorption of fat.
Other Short-term Studies
Adults
In several studies of up to 6-weeks duration, the effects of therapeutic doses of XENICAL on gastrointestinal and systemic physiological processes were assessed in normal weight and obese subjects. Postprandial cholecystokinin plasma concentrations were lowered after multiple doses of XENICAL in two studies but not significantly different from placebo in two other experiments. There were no clinically significant changes observed in gallbladder motility, bile composition or lithogenicity, or colonic cell proliferation rate, and no clinically significant reduction of gastric emptying time or gastric acidity. In addition, no effects on plasma triglyceride levels or systemic lipases were observed with the administration of XENICAL in these studies. In a 3-week study of 28 healthy male volunteers, XENICAL (120 mg three times a day) did not significantly affect the balance of calcium, magnesium, phosphorus, zinc, copper, and iron.
Pediatrics
In a 3-week study of 32 obese adolescents aged 12 to 16 years, XENICAL (120 mg three times a day) did not significantly affect the balance of calcium, magnesium, phosphorus, zinc, or copper. The iron balance was decreased by 64.7 µmole/24 hours and 40.4 µmole/24 hours in XENICAL and placebo treatment groups, respectively.
12.3. Pharmacokinetics
Absorption
Systemic exposure to orlistat is minimal. Following oral dosing with 360 mg 14C-orlistat, plasma radioactivity peaked at approximately 8 hours; plasma concentrations of intact orlistat were near the limits of detection (<5 ng/mL). In therapeutic studies involving monitoring of plasma samples, detection of intact orlistat in plasma was sporadic and concentrations were low (<10 ng/mL or 0.02 µM), without evidence of accumulation, and consistent with minimal absorption.
Distribution
In vitro orlistat was >99% bound to plasma proteins (lipoproteins and albumin were major binding proteins). Orlistat minimally partitioned into erythrocytes.
Metabolism
Based on an oral 14C-orlistat mass balance study in obese patients, two metabolites, M1 ((the hydrolyzed β-lactone ring product of orlistat) and M3 (sequential metabolite after M1’s cleavage of the N-formyl leucine side-chain), accounted for approximately 42% of total radioactivity in plasma. M1 and M3 have an open β-lactone ring and extremely weak lipase inhibitory activity (1000- and 2500-fold less than orlistat, respectively). In view of this low inhibitory activity and the low plasma levels at the therapeutic dose (average of 26 ng/mL and 108 ng/mL for M1 and M3, respectively, 2 to 4 hours after a dose), these metabolites are considered pharmacologically inconsequential. The primary metabolite M1 had a short half-life (approximately 3 hours) whereas the secondary metabolite M3 eliminated at a slower rate (half-life approximately 13.5 hours).
Elimination
Following a single oral dose of 360 mg 14C-orlistat in both normal weight and obese subjects, fecal excretion of the unabsorbed drug was found to be the major route of elimination. Orlistat and its M1 and M3 metabolites were also subject to biliary excretion. Approximately 97% of the administered radioactivity was excreted in feces; 83% of that was found to be unchanged orlistat. The cumulative renal excretion of total radioactivity was <2% of the given dose of 360 mg 14C-orlistat. The time to reach complete excretion (fecal plus urinary) was 3 to 5 days. The disposition of orlistat appeared to be similar between normal weight and obese subjects. Based on limited data, the half-life of the absorbed orlistat is in the range of 1 to 2 hours.
Specific Populations
No pharmacokinetic study was conducted for specific populations such as geriatric, different races, and patients with renal and hepatic impairment.
Drug Interactions
Alcohol
In a multiple-dose study in 30 normal-weight subjects, coadministration of XENICAL and 40 grams of alcohol (e.g., approximately 3 glasses of wine) did not result in alteration of alcohol pharmacokinetics, orlistat pharmacodynamics (fecal fat excretion), or systemic exposure to orlistat.
Amiodarone
In a pharmacokinetic study conducted in healthy volunteers who received 120 mg orlistat three times daily for 13 days and a single dose of 120 mg orlistat on the morning of Day 14 co-administered with a single dose of 1200 mg amiodarone on Day 4, a 23–27% reduction in the systemic exposure to amiodarone and desethylamiodarone was observed [see Drug Interactions (7.5)]. The effect of commencing orlistat treatment in patients on stable amiodarone therapy has not been studied.
Cyclosporine
In a multiple-dose study, coadministration of 50 mg cyclosporine twice daily with 120 mg XENICAL three times daily decreased cyclosporine AUC and Cmax by 31% and 25%, respectively. In the same study, administration of 50 mg cyclosporine twice daily three hours after the administration of 120 mg XENICAL three times daily decreased cyclosporine AUC and Cmax by 17% and 4%, respectively.
Digoxin
In 12 normal-weight subjects receiving XENICAL 120 mg three times a day for 6 days, XENICAL did not alter the pharmacokinetics of a single dose of digoxin.
Fat-soluble Vitamin Supplements and Analogues
A pharmacokinetic interaction study showed a 30% reduction in beta-carotene supplement absorption when concomitantly administered with XENICAL. XENICAL inhibited absorption of a vitamin E acetate supplement by approximately 60%. The effect of XENICAL on the absorption of supplemental vitamin D, vitamin A, and nutritionally-derived vitamin K is not known at this time.
Glyburide
In 12 normal-weight subjects receiving orlistat 80 mg three times a day for 5 days, orlistat did not alter the pharmacokinetics or pharmacodynamics (blood glucose-lowering) of glyburide.
Nifedipine (extended-release tablets)
In 17 normal-weight subjects receiving XENICAL 120 mg three times a day for 6 days, XENICAL did not alter the bioavailability of nifedipine (extended-release tablets).
Oral Contraceptives
In 20 normal-weight female subjects, the treatment of XENICAL 120 mg three times a day for 23 days resulted in no changes in the ovulation-suppressing action of oral contraceptives.
Phenytoin
In 12 normal-weight subjects receiving XENICAL 120 mg three times a day for 7 days, XENICAL did not alter the pharmacokinetics of a single 300-mg dose of phenytoin.
Pravastatin
In a 2-way crossover study of 24 normal-weight, mildly hypercholesterolemic patients receiving XENICAL 120 mg three times a day for 6 days, XENICAL did not affect the pharmacokinetics of pravastatin.
Warfarin
In 12 normal-weight subjects, administration of XENICAL 120 mg three times a day for 16 days did not result in any change in either warfarin pharmacokinetics (both R- and S-enantiomers) or pharmacodynamics (prothrombin time and serum Factor VII). Although undercarboxylated osteocalcin, a marker of vitamin K nutritional status, was unaltered with XENICAL administration, vitamin K levels tended to decline in subjects taking XENICAL. Therefore, as vitamin K absorption may be decreased with XENICAL, patients on chronic stable doses of warfarin who are prescribed XENICAL should be monitored closely for changes in coagulation parameters.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in rats and mice did not show a carcinogenic potential for orlistat at doses up to 1000 mg/kg/day and 1500 mg/kg/day, respectively. For mice and rats, these doses are 38 and 46 times the daily human dose calculated on an area under concentration vs time curve basis of total drug-related material.
Orlistat had no detectable mutagenic or genotoxic activity as determined by the Ames test, a mammalian forward mutation assay (V79/HPRT), an in vitro clastogenesis assay in peripheral human lymphocytes, an unscheduled DNA synthesis assay (UDS) in rat hepatocytes in culture, and an in vivo mouse micronucleus test.
When given to rats at a dose of 400 mg/kg/day in a fertility and reproduction study, orlistat had no observable adverse effects. This dose is 12 times the daily human dose calculated on a body surface area (mg/m²) basis.
14. Clinical Studies
The long-term effects of XENICAL on morbidity and mortality associated with obesity have not been established.
The effects of XENICAL on weight loss, weight maintenance, and weight regain and on a number of comorbidities (e.g., type 2 diabetes, lipids, blood pressure) were assessed in the 4-year XENDOS study and in seven long-term (1- to 2-years duration) multicenter, double-blind, placebo-controlled clinical trials. During the first year of therapy, the studies of 2-year duration assessed weight loss and weight maintenance. During the second year of therapy, some studies assessed continued weight loss and weight maintenance and others assessed the effect of XENICAL on weight regain. These studies included over 2800 patients treated with XENICAL and 1400 patients treated with placebo (age range 17-78 years, 80.2% women, 91.0% Caucasians, 5.7% Blacks, 2.3% Hispanics, 0.9% Other). The majority of these patients had obesity-related risk factors and comorbidities. In the XENDOS study, which included 3304 patients (age range 30-58 years, 55% women, 99% Caucasians, 1% other), the time to onset of type 2 diabetes was assessed in addition to weight management. In all these studies, treatment with XENICAL and placebo designates treatment with XENICAL plus diet and placebo plus diet, respectively.
During the weight loss and weight maintenance period, a well-balanced, reduced-calorie diet that was intended to result in an approximate 20% decrease in caloric intake and provide 30% of calories from fat was recommended to all patients. In addition, all patients were offered nutritional counseling.
14.1 One-year Results: Weight Loss, Weight Maintenance, and Risk Factors
Pooled data from five clinical trials indicated that the overall mean weight loss from randomization to the end of 1 year of treatment in the intent-to-treat population was 13.4 lbs in the patients treated with XENICAL and 5.8 lbs in the placebo-treated patients. After 1 year of treatment, the mean percent weight loss difference between XENICAL-treated patients and placebo-treated patients was 3%. One thousand seventy two (69%) patients treated with XENICAL and 701 (63%) patients treated with placebo completed 1 year of treatment. Of the patients who completed 1 year of treatment, 57% of the patients treated with XENICAL (120 mg three times a day) and 31% of the placebo-treated patients lost at least 5% of their baseline body weight.
The percentages of patients achieving ≥5% and ≥10% weight loss after 1 year in five large multicenter studies for the intent-to-treat populations are presented in Table 6.
Table 6. Percentage of Patients Losing ≥5% and ≥10% of Body Weight From Randomization After 1-Year Treatment*:
| Study No. | Intent-to-Treat Population† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥5% Weight Loss | ≥10% Weight Loss | ||||||||||
| XENICAL | n | Placebo | n | p-value | XENICAL | n | Placebo | n | p-value | ||
| 14119B | 35.5% | 110 | 21.3% | 108 | 0.021 | 16.4% | 110 | 6.5% | 108 | 0.022 | |
| 14119C | 54.8% | 343 | 27.4% | 340 | <0.001 | 24.8% | 343 | 8.2% | 340 | <0.001 | |
| 14149 | 50.6% | 241 | 26.3% | 236 | <0.001 | 22.8% | 241 | 11.9% | 236 | 0.02 | |
| 14161‡ | 37.1% | 210 | 16.0% | 212 | <0.001 | 19.5% | 210 | 3.8% | 212 | <0.001 | |
| 14185 | 42.6% | 657 | 22.4% | 223 | <0.001 | 17.7% | 657 | 9.9% | 223 | 0.006 | |
The diet utilized during year 1 was a reduced-calorie diet.
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Last observation carried forward
‡ All studies, with the exception of 14 161, were conducted at centers specialized in treating obesity and complications of obesity. Study 14 161 was conducted with primary care physicians.
The relative changes in risk factors associated with obesity following 1 year of therapy with XENICAL and placebo are presented for the population as a whole and for the population with abnormal values at randomization.
Population as a Whole
The changes in metabolic, cardiovascular and anthropometric risk factors associated with obesity based on pooled data for five clinical studies, regardless of the patient’s risk factor status at randomization, are presented in Table 7. One year of therapy with XENICAL resulted in relative improvement in several risk factors.
Table 7. Mean Change in Risk Factors From Randomization Following 1-Year Treatment* Population as a Whole:
| Risk Factor | XENICAL 120 mg† | Placebo† |
|---|---|---|
| Metabolic: | ||
| Total Cholesterol | -2.0% | +5.0% |
| LDL-Cholesterol | -4.0% | +5.0% |
| HDL-Cholesterol | +9.3% | +12.8% |
| LDL/HDL | -0.37 | -0.20 |
| Triglycerides | +1.34% | +2.9% |
| Fasting Glucose, mmol/L | -0.04 | +0.0 |
| Fasting Insulin, pmol/L | -6.7 | +5.2 |
| Cardiovascular: | ||
| Systolic Blood Pressure, mm Hg | -1.01 | +0.58 |
| Diastolic Blood Pressure, mm Hg | -1.19 | +0.46 |
| Anthropometric: | ||
| Waist Circumference, cm | -6.45 | -4.04 |
| Hip Circumference, cm | -5.31 | -2.96 |
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Intent-to-treat population at week 52, observed data based on pooled data from 5 studies
Population With Abnormal Risk Factors at Randomization
The changes from randomization following 1-year treatment in the population with abnormal lipid levels (LDL ≥130 mg/dL, LDL/HDL ≥3.5, HDL <35 mg/dL) were greater for XENICAL compared to placebo with respect to LDL-cholesterol (-7.83% vs +1.14%) and the LDL/HDL ratio (-0.64 vs -0.46). HDL increased in the placebo group by 20.1% and in the XENICAL group by 18.8%. In the population with abnormal blood pressure at baseline (systolic BP ≥140 mm Hg), the change in SBP from randomization to 1 year was greater for XENICAL (-10.89 mm Hg) than placebo (-5.07 mm Hg). For patients with a diastolic blood pressure ≥90 mm Hg, XENICAL patients decreased by -7.9 mm Hg while the placebo patients decreased by -5.5 mm Hg. Fasting insulin decreased more for XENICAL than placebo (-39 vs -16 pmol/L) from randomization to 1 year in the population with abnormal baseline values (≥120 pmol/L). A greater reduction in waist circumference for XENICAL vs placebo (-7.29 vs -4.53 cm) was observed in the population with abnormal baseline values (≥100 cm).
14.2 Effect on Weight Regain
Three studies were designed to evaluate the effects of XENICAL compared to placebo in reducing weight regain after a previous weight loss achieved following either diet alone (one study, 14302) or prior treatment with XENICAL (two studies, 14119C and 14185). The diet utilized during the 1-year weight regain portion of the studies was a weight-maintenance diet, rather than a weight-loss diet, and patients received less nutritional counseling than patients in weight-loss studies. For studies 14119C and 14185, patients' previous weight loss was due to 1 year of treatment with XENICAL in conjunction with a mildly hypocaloric diet. Study 14302 was conducted to evaluate the effects of 1 year of treatment with XENICAL on weight regain in patients who had lost 8% or more of their body weight in the previous 6 months on diet alone.
In study 14119C, patients treated with placebo regained 52% of the weight they had previously lost while the patients treated with XENICAL regained 26% of the weight they had previously lost (p<0.001). In study 14185, patients treated with placebo regained 63% of the weight they had previously lost while the patients treated with XENICAL regained 35% of the weight they had lost (p<0.001). In study 14302, patients treated with placebo regained 53% of the weight they had previously lost while the patients treated with XENICAL regained 32% of the weight that they had lost (p<0.001).
14.3 Two-year Results: Long-term Weight Control and Risk Factors
The treatment effects of XENICAL were examined for 2 years in four of the five 1-year weight management clinical studies previously discussed (see Table 6). At the end of year 1, the patients' diets were reviewed and changed where necessary. The diet prescribed in the second year was designed to maintain patient’s current weight. XENICAL was shown to be more effective than placebo in long-term weight control in four large, multicenter, 2-year double-blind, placebo-controlled studies.
Pooled data from four clinical studies indicate that 74% of all patients treated with 120 mg three times a day of XENICAL and 76% of patients treated with placebo completed 2 years of the same therapy. Pooled data from four clinical studies indicate that the mean weight loss difference between XENICAL 120 mg three times a day and placebo treatment groups at year 2 in those patients who completed 1 year of treatment (ITT LOCF) was 3%. In the same studies cited in the One-year Results (see Table 6), the percentages of patients achieving a ≥5% and ≥10% weight loss after 2 years are shown in Table 8.
Table 8. Percentage of Patients Losing ≥5% and ≥10% of Body Weight From Randomization After 2-Year Treatment*:
| Study No. | Intent-to-Treat Population† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥5% Weight Loss | ≥10% Weight Loss | ||||||||||
| XENICAL | n | Placebo | n | p-value | XENICAL | n | Placebo | n | p-value | ||
| 14119C | 45.1% | 133 | 23.6% | 123 | <0.001 | 24.8% | 133 | 6.5% | 123 | <0.001 | |
| 14149 | 43.3% | 178 | 27.2% | 158 | 0.002 | 18.0% | 178 | 9.5% | 158 | 0.025 | |
| 14161 ?footnote? | 25.0% | 148 | 15.0% | 113 | 0.049 | 16.9% | 148 | 3.5% | 113 | 0.001 | |
| 14185 | 34.0% | 147 | 27.9% | 122 | 0.279 | 17.7% | 147 | 11.5% | 122 | 0.154 | |
The diet utilized during year 2 was designed for weight maintenance and not weight loss.
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Last observation carried forward
‡ All studies, with the exception of 14 161, were conducted at centers specializing in treating obesity or complications of obesity. Study 14 161 was conducted with primary care physicians.
The relative changes in risk factors associated with obesity following 2 years of therapy were also assessed in the population as a whole and the population with abnormal risk factors at randomization.
Population as a Whole
The relative differences in risk factors between treatment with XENICAL and placebo were similar to the results following 1 year of therapy for total cholesterol, LDL-cholesterol, LDL/HDL ratio, triglycerides, fasting glucose, fasting insulin, diastolic blood pressure, waist circumference, and hip circumference. The relative differences between treatment groups for HDL cholesterol and systolic blood pressure were less than that observed in the year one results.
Population With Abnormal Risk Factors at Randomization
The relative differences in risk factors between treatment with XENICAL and placebo were similar to the results following 1 year of therapy for LDL- and HDL-cholesterol, triglycerides, fasting insulin, diastolic blood pressure, and waist circumference. The relative differences between treatment groups for LDL/HDL ratio and isolated systolic blood pressure were less than that observed in the year one results.
14.4 Four-year Results: Long-term Weight Control and Risk Factors
In the 4-year double-blind, placebo-controlled XENDOS study, the effects of XENICAL in delaying the onset of type 2 diabetes and on body weight were compared to placebo in 3304 obese patients who had either normal or impaired glucose tolerance at baseline. Thirty-four percent of the 1655 patients who were randomized to the placebo group and 52% of the 1649 patients who were randomized to the XENICAL group completed the 4-year study.
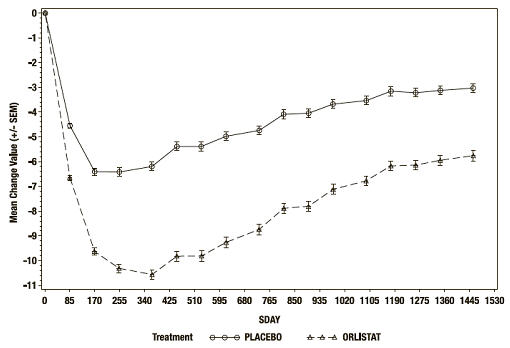
At the end of the study, the mean percent weight loss in the placebo group was -2.75% compared with -5.17% in the XENICAL group (p<0.001) (see Figure 2). Forty-five percent of the placebo patients and 73% of the XENICAL patients lost ≥5% of their baseline body weight, and 21% of the placebo patients and 41% of the XENICAL patients lost ≥10% of their baseline body weight following the first year of treatment. Following 4 years of treatment, 28% of the placebo patients and 45% of the XENICAL patients lost ≥5% of their baseline body weight and 10% of the placebo patients and 21% of the XENICAL patients lost ≥10% of their baseline body weight. After 4 years of treatment, the mean % difference in weight loss between XENICAL treated patients and placebo was 2.5%.
Figure 2. Mean Change from Baseline Body Weight (Kgs) Over Time*:
* ITT LOCF study population
The relative changes from baseline in risk factors associated with obesity following 4 years of therapy were assessed in the XENDOS study population (see Table 9).
Table 9. Mean Change in Risk Factors From Randomization Following 4-Years Treatment*:
| Risk Factor | XENICAL 120 mg† | Placebo† |
|---|---|---|
| Metabolic: | ||
| Total Cholesterol | -7.02% | -2.03% |
| LDL-Cholesterol | -11.66% | -3.85% |
| HDL-Cholesterol | +5.92% | +7.01% |
| LDL/HDL | -0.53 | -0.33 |
| Triglycerides | +3.64% | +1.30 |
| Fasting Glucose, mmol/L | +0.12 | +0.23 |
| Fasting Insulin, pmol/L | -24.93 | -15.71 |
| Cardiovascular: | ||
| Systolic Blood Pressure, mm Hg | -4.12 | -2.60 |
| Diastolic Blood Pressure, mm Hg | -1.93 | -0.87 |
| Anthropometric: | ||
| Waist Circumference, cm | -5.78 | -3.99 |
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Intent-to-treat population
Onset of Type 2 Diabetes in Obese Patients
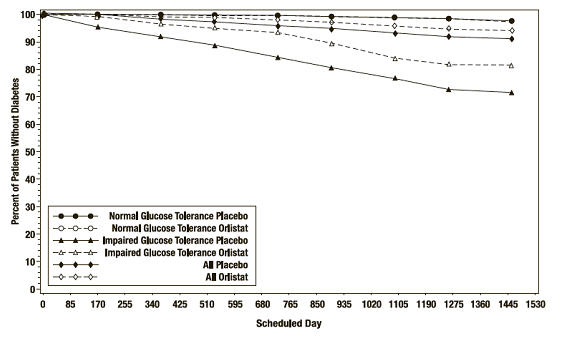
In the XENDOS trial, in the overall population, XENICAL delayed the onset of type 2 diabetes such that at the end of four years of treatment the cumulative incidence rate of diabetes was 8.3% for the placebo group compared to 5.5% for the XENICAL group, p=0.01 (see Table 10). This finding was driven by a statistically-significant reduction in the incidence of developing type 2 diabetes in those patients who had impaired glucose tolerance at baseline (Table 10 and Figure 3). XENICAL did not reduce the risk for the development of diabetes in patients with normal glucose tolerance at baseline.
The effect of XENICAL to delay the onset of type 2 diabetes in obese patients with IGT is presumably due to weight loss, and not to any independent effects of the drug on glucose or insulin metabolism. The effect of XENICAL on weight loss is adjunctive to diet and exercise.
Table 10. Incidence Rate of Diabetes at Year 4 by OGTT Status at Baseline*:
| OGTT at Baseline | Normal | Impaired | All | |||
|---|---|---|---|---|---|---|
| Treatment | Placebo | XENICAL | Placebo | XENICAL | Placebo | XENICAL |
| Number of patients* | 1148 | 1235 | 324 | 337 | 1472 | 1572 |
| # pts developing diabetes | 16 | 21 | 62 | 48 | 78 | 69 |
| Life table rate† | 2.1% | 1.7% | 27.2% | 18.7% | 8.3% | 5.5% |
| Observed percent | 1.4% | 1.7% | 19.1% | 14.2% | 5.3% | 4.4% |
| Absolute risk reduction | ||||||
| Life table | 0.4% | 8.5% | 2.8% | |||
| Observed | -0.3% | 4.9% | 0.9% | |||
| Relative risk reduction‡ | 8% | 42% | 34% | |||
| p-value | 0.79 | <0.01 | 0.01 | |||
* Based on patients with a baseline and at least one follow-up OGTT measurement, ITT LOCF study population.
† Rate adjusted for dropouts
‡ Computed as (1- hazard ratio)
Figure 3. Percentage of Patients Without Diabetes Over Time:
14.5 Study of Patients With Type 2 Diabetes
A 1-year double-blind, placebo-controlled study in type 2 diabetics (N=321) stabilized on sulfonylureas was conducted. Thirty percent of patients treated with XENICAL achieved at least a 5% or greater reduction in body weight from randomization compared to 13% of the placebo-treated patients (p<0.001). Table 11 describes the changes over 1 year of treatment with XENICAL compared to placebo, in sulfonylurea usage and dose reduction as well as in hemoglobin HbA1c, fasting glucose, and insulin.
Table 11. Mean Changes in Body Weight and Glycemic Control From Randomization Following 1-Year Treatment in Patients With Type 2 Diabetes:
| XENICAL 120 mg* (n=162) | Placebo* (n=159) | Statistical Significance | |
|---|---|---|---|
| % patients who discontinued dose of oral sulfonylurea | 11.7% | 7.5% | † |
| % patients who decreased dose of oral sulfonylurea | 31.5% | 21.4% | |
| Average reduction in sulfonylurea medication dose | -22.8% | -9.1% | † |
| Body weight change (lbs) | -8.9 | -4.2 | † |
| HbA1c | -0.18% | +0.28% | † |
| Fasting glucose, mmol/L | -0.02 | +0.54 | † |
| Fasting insulin, pmol/L | -19.68 | -18.02 | ns |
Statistical significance based on intent-to-treat population, last observation carried forward.
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Statistically significant (p≤0.05) based on intent-to-treat, last observation carried forward ns nonsignificant, p>0.05
Statistical significance based on intent-to-treat population, last observation carried forward.
In addition, XENICAL (n=162) compared to placebo (n=159) was associated with significant lowering for total cholesterol (-1.0% vs +9.0%, p≤0.05), LDL-cholesterol (-3.0% vs +10.0%, p≤0.05), LDL/HDL ratio (-0.26 vs -0.02, p≤0.05) and triglycerides (+2.54% vs +16.2%, p≤0.05), respectively. For HDL cholesterol, there was a +6.49% increase on XENICAL and +8.6% increase on placebo, p>0.05. Systolic blood pressure increased by +0.61 mm Hg on XENICAL and increased by +4.33 mm Hg on placebo, p>0.05. Diastolic blood pressure decreased by -0.47 mm Hg for XENICAL and by -0.5 mm Hg for placebo, p>0.05.
14.6 Glucose Tolerance in Obese Patients
Two-year studies that included oral glucose tolerance tests were conducted in obese patients not previously diagnosed or treated for type 2 diabetes and whose baseline oral glucose tolerance test (OGTT) status at randomization was either normal, impaired, or diabetic.
The progression from a normal OGTT at randomization to a diabetic or impaired OGTT following 2 years of treatment with XENICAL (n=251) or placebo (n=207) were compared. Following treatment with XENICAL, 0.0% and 7.2% of the patients progressed from normal to diabetic and normal to impaired, respectively, compared to 1.9% and 12.6% of the placebo treatment group, respectively.
In patients found to have an impaired OGTT at randomization, the percent of patients improving to normal or deteriorating to diabetic status following 1 and 2 years of treatment with XENICAL compared to placebo are presented. After 1 year of treatment, 45.8% of the placebo patients and 73% of the XENICAL patients had a normal oral glucose tolerance test while 10.4% of the placebo patients and 2.6% of the XENICAL patients became diabetic. After 2 years of treatment, 50% of the placebo patients and 71.7% of the XENICAL patients had a normal oral glucose tolerance test while 7.5% of placebo patients were found to be diabetic and 1.7% of XENICAL patients were found to be diabetic after treatment.
14.7 Pediatric Clinical Studies
The effects of XENICAL on body mass index (BMI) and weight loss were assessed in a 54-week multicenter, double-blind, placebo-controlled study in 539 obese adolescents (357 receiving XENICAL 120 mg three times a day, 182 receiving placebo), aged 12 to 16 years. All study participants had a baseline BMI that was 2 units greater than the US weighted mean for the 95th percentile based on age and gender. Body mass index was the primary efficacy parameter because it takes into account changes in height and body weight, which occur in growing children.
During the study, all patients were instructed to take a multivitamin containing fat-soluble vitamins at least 2 hours before or after ingestion of XENICAL. Patients were also maintained on a well-balanced, reduced-calorie diet that was intended to provide 30% of calories from fat. In addition, all patients were placed on a behavior modification program and offered exercise counseling.
Approximately 65% of patients in each treatment group completed the study.
Following one year of treatment, BMI decreased by an average of 0.55 kg/m² in the XENICAL-treated patients and increased by an average of 0.31 kg/m² in the placebo-treated patients (p=0.001).
The percentages of patients achieving ≥5% and ≥10% reduction in BMI and body weight after 52 weeks of treatment for the intent-to-treat population are presented in Table 12.
Table 12. Percentages of Patients with ≥5% and ≥10% Decrease in Body Mass Index and Body Weight After 1-Year Treatment* (Protocol NM16189):
| Intent-to-Treat Population† | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≥5% Decrease | ≥10% Decrease | |||||||
| XENICAL | n | Placebo | n | XENICAL | n | Placebo | n | |
| BMI | 26.5% | 347 | 15.7% | 178 | 13.3% | 347 | 4.5% | 178 |
| Body Weight | 19.0% | 348 | 11.7% | 180 | 9.5% | 348 | 3.3% | 180 |
* Treatment designates XENICAL 120 mg three times a day plus diet or placebo plus diet
† Last observation carried forward
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.