Angiox 250mg powder for concentrate for solution for injection or infusion Ref.[2569] Active ingredients: Bivalirudin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2012 Publisher: The Medicines Company UK Ltd 115L Milton Park Abingdon Oxfordshire OX14 4SA UNITED KINGDOM
Contraindications
Angiox is contraindicated in patients with:
- a known hypersensitivity to the active substance or to any of the excipients, or to hirudins
- active bleeding or increased risk of bleeding because of haemostasis disorders and/or irreversible coagulation disorders
- severe uncontrolled hypertension
- subacute bacterial endocarditis
- severe renal impairment (GFR<30 ml/min) and in dialysis-dependent patients.
Special warnings and precautions for use
Angiox is not intended for intramuscular use. Do not administer intramuscularly.
Haemorrhage
Patients must be observed carefully for symptoms and signs of bleeding during treatment particularly if bivalirudin is combined with another anticoagulant (see section 4.5). Although most bleeding associated with bivalirudin occurs at the site of arterial puncture in patients undergoing PCI, haemorrhage can occur at any site during therapy. Unexplained decreases in haematocrit, haemoglobin or blood pressure may indicate haemorrhage. Treatment should be stopped if bleeding is observed or suspected.
There is no known antidote to bivalirudin but its effect wears off quickly (T½ is 35 to 40 minutes).
Co-administration with platelet inhibitors or anti-coagulants
Combined use of anti-coagulant medicines can be expected to increase the risk of bleeding (see section 4.5). When bivalirudin is combined with a platelet inhibitor or an anti-coagulant medicine, clinical and biological parameters of haemostasis should be regularly monitored.
In patients taking warfarin who are treated with bivalirudin, International Normalised Ratio (INR) monitoring should be considered to ensure that it returns to pre-treatment levels following discontinuation of bivalirudin treatment.
Hypersensitivity
Allergic type hypersensitivity reactions were reported uncommonly (≥1/1,000 to ≤1/100) in clinical trials. Necessary preparations should be made to deal with this. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalised urticaria, tightness of chest, wheezing, hypotension and anaphylaxis. In the case of shock, the current medical standards for shock treatment should be applied. Anaphylaxis, including anaphylactic shock with fatal outcome has been reported very rarely (≤1/10,000) in post-marketing experience (see section 4.8).
Treatment-emergent positive bivalirudin antibodies are rare and have not been associated with clinical evidence of allergic or anaphylactic reactions. Caution should be exercised in patients previously treated with lepirudin who had developed lepirudin antibodies.
Acute stent thrombosis
Acute stent thrombosis (<24 hours) has been observed in patients with STEMI undergoing primary PCI and has been managed by Target Vessel Revascularisation (TVR) (see sections 4.8 and 5.1). Patients should remain for at least 24 hours in a facility capable of managing ischaemic complications and should be carefully monitored following primary PCI for signs and symptoms consistent with myocardial ischaemia.
Brachytherapy
Intra-procedural thrombus formation has been observed during gamma brachytherapy procedures with Angiox.
Angiox should be used with caution during beta brachytherapy procedures.
Interaction with other medicinal products and other forms of interaction
Interaction studies have been conducted with platelet inhibitors, including acetylsalicylic acid, ticlopidine, clopidogrel, abciximab, eptifibatide, or tirofiban. The results do not suggest pharmacodynamic interactions with these medicinal products.
From the knowledge of their mechanism of action, combined use of anti-coagulant medicinal products (heparin, warfarin, thrombolytics or antiplatelet agents) can be expected to increase the risk of bleeding.
In any case, when bivalirudin is combined with a platelet inhibitor or an anticoagulant medicine, clinical and biological parameters of haemostasis should be regularly monitored.
Pregnancy and lactation
Pregnancy
There are no or limited data from the use of bivalirudin in pregnant women. Animal studies are insufficient with respect to effects on pregnancy, embryonal/foetal development, parturition or post-natal development (see section 5.3).
Angiox should not be used during pregnancy unless the clinical condition of the woman requires treatment with bivalirudin.
Breastfeeding
It is unknown whether bivalirudin is excreted in human milk. Angiox should be administered with caution in breast-feeding mothers.
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
Undesirable effects
Adverse reactions for bivalirudin from HORIZONS, ACUITY, REPLACE-2 trials and post-marketing experience are listed by system organ class in Table 1.
In all clinical studies bleeding data were collected separately from adverse drug reactions and are summarised in Table 6 together with the bleeding definitions used for each study.
The HORIZONS Trial (Patients with STEMI undergoing primary PCI)
The following data are based on a clinical study of bivalirudin in patients with STEMI undergoing primary PCI; 1,800 patients were randomised to bivalirudin alone, 1,802 were randomised to heparin plus GP IIb/IIIa inhibitor. Serious adverse reactions were reported more frequently in the heparin plus GP IIb/IIIa group than the bivalirudin treated group.
A total of 55.1% of patients receiving bivalirudin experienced at least one adverse event and 8.7% experienced an adverse drug reaction. Adverse drug reactions for bivalirudin are listed by system organ class in Table 1.The incidence of stent thrombosis within the first 24 hours was 1.5% in patients receiving bivalirudin versus 0.3% in patients receiving UFH plus GP IIb/IIIa inhibitor (p=0.0002). Two deaths occurred after acute stent thrombosis, 1 in each arm of the study. The incidence of stent thrombosis between 24 hours and 30 days was 1. 2% in patients receiving bivalirudin versus 1.9% in patients receiving UFH plus GP IIb/IIIa inhibitor (p=0.1553). A total of 17 deaths occurred after subacute stent thrombosis, 3 in the bivalirudin arm and 14 in the UFH plus GP IIb/IIIa arm. There was no statistically significant difference in the rates of stent thrombosis between treatment arms at 30 days (p=0.3257) and 1 year (p=0.7754).
Platelets, bleeding and clotting: In the HORIZONS study both major and minor bleeding occurred commonly (≥1/100 and <1/10). The incidence of major and minor bleeding was significantly less in patients treated with bivalirudin versus patients treated with heparin plus a GP IIb/IIIa inhibitor. The incidence of major bleeding is shown in Table 6. Major bleeding occurred most frequently at the sheath puncture site. The most frequent event was a haematoma <5 cm at puncture site.
In the HORIZONS study, thrombocytopenia was reported in 26 (1. 6%) of bivalirudin-treated patients and in 67 (3.9%) of patients treated with heparin plus a GP IIb/IIIa inhibitor. All of these bivalirudin-treated patients received concomitant aspirin, all but 1 received clopidogrel and 15 also received a GP IIb/IIIa inhibitor.
The ACUITY Trial (Patients with unstable angina/non-ST segment elevated myocardial infarction (UA/NSTEMI))
The following data are based on a clinical study of bivalirudin in 13,819 patients with ACS; 4,612 were randomised to bivalirudin alone, 4,604 were randomised to bivalirudin plus GP IIb/IIIa inhibitor and 4,603 were randomised to either unfractionated heparin or enoxaparin plus GP IIb/IIIa inhibitor. Adverse reactions were more frequent in females and in patients more than 65 years of age in both the bivalirudin and the heparin-treated comparator groups compared to male or younger patients.
Approximately 23.3% of patients receiving bivalirudin experienced at least one adverse event and 2.1% experienced an adverse reaction. Adverse event reactions for bivalirudin are listed by system organ class in Table 1.
Platelets, bleeding and clotting: In ACUITY, bleeding data were collected separately from adverse reactions.
ACUITY major bleeding was defined as any one of the following: intracranial, retroperitoneal, intraocular, access site haemorrhage requiring radiological or surgical intervention, ≥5 cm diameter haematoma at puncture site, reduction in haemoglobin concentration of ≥4 g/dl without an overt source of bleeding, reduction in haemoglobin concentration of ≥3 g/dl with an overt source of bleeding, re-operation for bleeding or use of any blood product transfusion. Minor bleeding was defined as any observed bleeding event that did not meet the criteria as major. Minor bleeding occurred very commonly (≥1/10) and major bleeding occurred commonly (≥1/100 and <1/10).
Major bleeding rates are shown in Table 6 for the IIT population and Table 7 for the per protocol population (patients receiving clopidogrel and aspirin). Both major and minor bleeds were significantly less frequent with bivalirudin alone than the heparin plus GP IIb/IIIa inhibitor and bivalirudin plus GP IIb/IIIa inhibitor groups. Similar reductions in bleeding were observed in patients who were switched to bivalirudin from heparin-based therapies (N = 2,078).
Major bleeding occurred most frequently at the sheath puncture site. Other less frequently observed bleeding sites with greater than 0.1% (uncommon) bleeding included “other” puncture site, retroperitoneal, gastrointestinal, ear, nose or throat.
Thrombocytopenia was reported in 10 bivalirudin-treated patients participating in the ACUITY study (0.1%). The majority of these patients received concomitant acetylsalicylic acid and clopidogrel, and 6 out of the 10 patients also received a GP IIb/IIIa inhibitor. Mortality among these patients was nil.
The REPLACE-2 Trial (Patients undergoing PCI)
The following data is based on a clinical study of bivalirudin in 6,000 patients undergoing PCI, half of whom were treated with bivalirudin (REPLACE-2). Adverse events were more frequent in females and in patients more than 65 years of age in both the bivalirudin and the heparin-treated comparator groups compared to male or younger patients.
Approximately 30% of patients receiving bivalirudin experienced at least one adverse event and 3% experienced an adverse reaction. Adverse reactions for bivalirudin are listed by system organ class in Table 1.
Platelets, bleeding and clotting: In REPLACE-2, bleeding data were collected separately from adverse events. Major bleeding rates for the intent-to-treat trial population is shown in Table 6.
Major bleeding was defined as the occurrence of any of the following: intracranial haemorrhage, retroperitoneal haemorrhage, blood loss leading to a transfusion of at least two units of whole blood or packed red blood cells, or bleeding resulting in a haemoglobin drop of more than 3 g/dl, or a fall in haemoglobin greater than 4 g/dl (or 12% of haematocrit) with no bleeding site identified. Minor haemorrhage was defined as any observed bleeding event that did not meet the criteria for a major haemorrhage. Minor bleeding occurred very commonly (≥1/10) and major bleeding occurred commonly (≥1/100 and <1/10).
Both minor and major bleeds were significantly less frequent with bivalirudin than the heparin plus GP IIb/IIIa inhibitor comparator group. Major bleeding occurred most frequently at the sheath puncture site. Other less frequently observed bleeding sites with greater than 0.1% (uncommon) bleeding included “other” puncture site, retroperitoneal, gastrointestinal, ear, nose or throat.
In REPLACE-2 thrombocytopenia occurred in 20 bivalirudin-treated patients (0.7%). The majority of these patients received concomitant aspirin and clopidogrel, and 10 out of 20 patients also received a GP IIb/IIIa inhibitor. Mortality among these patients was nil.
Table 1 – Adverse drug reactions for bivalirudin from HORIZONS, ACUITY, REPLACE-2 trials and post-marketing experience:
Blood and lymphatic system disorders
Common (≥1/100 to <1/10): Haemoglobin decreased
Uncommon (≥1/1,000 to <1/100): Thrombocytopenia, Anaemia
Rare (≥1/10,000 to <1/1,000): INR increased
Cardiac disorders
Rare (≥1/10,000 to <1/1,000): Myocardial infarction, Cardiac tamponade, Pericardial haemorrhage, Coronary artery thrombosis, Angina pectoris, Bradycardia, Ventricular tachycardia, Chest pain
Ear and labyrinth disorders
Rare (≥1/10,000 to <1/1,000): Ear haemorrhage
Eye disorders
Rare (≥1/10,000 to <1/1,000): Intraocular haemorrhage
Gastrointestinal disorders
Uncommon (≥1/1,000 to <1/100): Gastrointestinal haemorrhage (including haematemesis, malaena, oesophageal haemorrhage, anal haemorrhage), Retroperitoneal haemorrhage, Gingival haemorrhage, Nausea
Rare (≥1/10,000 to <1/1,000): Peritoneal haemorrhage, Retroperitoneal haematoma, Vomiting
General disorders and administration site conditions
Common (≥1/100 to <1/10): Access site haemorrhage, Vessel puncture site haematoma ≥5 cm, Vessel puncture site haematoma <5 cm
Rare (≥1/10,000 to <1/1,000): Injection site reactions (Injection site discomfort, Injection site pain, Puncture site reaction)
Injury, poisoning and procedural complications
Uncommon (≥1/1,000 to <1/100): Reperfusion injury (no or slow reflow), Contusion
Immune system disorders
Uncommon (≥1/1,000 to <1/100): Hypersensitivity, including anaphylactic reaction and shock, including reports with fatal outcome
Musculoskeletal and connective tissue disorders
Rare (≥1/10,000 to <1/1,000): Back pain, Groin pain
Nervous system disorders
Uncommon (≥1/1,000 to <1/100): Headache
Rare (≥1/10,000 to <1/1,000): Intracranial haemorrhage
Renal and urinary disorders
Uncommon (≥1/1,000 to <1/100): Haematuria
Respiratory, thoracic and mediastinal disorders
Uncommon (≥1/1,000 to <1/100): Epistaxis, Haemoptysis, Pharyngeal haemorrhage
Rare (≥1/10,000 to <1/1,000): Pulmonary haemorrhage, Dyspnoeaa
Skin and subcutaneous tissue disorders
Common (≥1/100 to <1/10): Ecchymosis
Rare (≥1/10,000 to <1/1,000): Rash, Urticaria
Vascular disorders
Very common (≥1/10): Minor haemorrhage at any site
Common (≥1/100 to <1/10): Major haemorrhage at any site including reports with fatal outcome
Uncommon (≥1/1,000 to <1/100): Haematoma, Hypotension
Rare (≥1/10,000 to <1/1,000): Coronary stent thrombosis including reports with fatal outcomec, Thrombosis including reports with fatal outcome, Arteriovenous fistula, Catheter thrombosis, Vascular pseudoaneurysm
Very rare ( <1/10,000): Compartment syndromea,b
a. ADRs identified in post-marketing experience
b. Compartment syndrome has been reported as a complication of forearm haematoma following administration of bivalirudin via the radial access route in post-marketing experience
c. Further detail regarding stent thrombosis is provided in section 4.8: The HORIZONS Trial (Patients with STEMI undergoing primary PCI). Please also refer to section 4.4 for instructions for monitoring acute stent thrombosis.
Incompatibilities
The following medicinal products should not be administered in the same intravenous line as bivalirudin since they result in haze formation, micro-particulate formation or gross precipitation; alteplase, amiodarone HCl, amphotericin B, chlorpromazine HCl, diazepam, prochlorperazine edisylate, reteplase, streptokinase and vancomycin HCl.
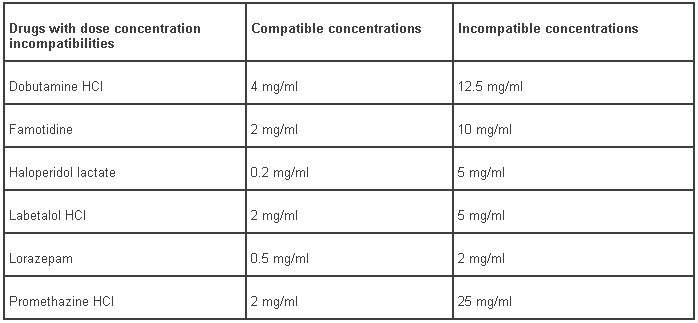
The following six drugs show dose-concentration incompatibilities with bivalirudin. Table 9 summarises compatible and incompatible concentrations of these compounds. The medicinal products incompatible with bivalirudin at higher concentrations are: dobutamine hydrochloride, famotidine, haloperidol lactate, labetalol hydrochloride, lorazepam and promethazine HCl.
Table 9 – Drugs with dose concentration incompatibilities to bivalirudin:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.