ARTESUNATE Powder for solution for injection Ref.[27495] Active ingredients: Artesunate
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Artesunate is an antimalarial drug [see Microbiology (12.4)].
12.2. Pharmacodynamics
Artesunate and DHA exposure-response relationships and their time course of pharmacodynamic responses are unknown.
Cardiac Electrophysiology
At the approved intravenous dose of 2.4 mg/kg Artesunate for Injection, artesunate and DHA do not cause large mean increases (i.e., 20 msec) in the QTc interval.
12.3. Pharmacokinetics
Following administration of 2.4 mg/kg Artesunate for Injection, artesunate is rapidly converted to DHA by blood esterases. The PK parameters of artesunate (AS) and DHA in patients with severe malaria following administration of multiple doses of 2.4 mg/kg Artesunate for Injection are shown in Table 2.
Table 2. Summary of Median (Range) Pharmacokinetic Parameters in Patients with Severe Malaria (N=14):
| PK Parameter | AS | DHA |
|---|---|---|
| Cmax (mcg/mL) | 3.3 (1.0-164) | 3.1 (1.7-9.5) |
| AUC (mcg-h/mL) | 0.7 (0.3-111.3) | 3.5 (2.2-6.3) |
| Distribution | ||
| Volume of Distribution (L) | 68.5 (0.2-818) | 59.7 (26-117) |
| Protein Binding | Approximately 93% | |
| Elimination | ||
| Half-life (hours) | 0.3 (0.1-1.8) | 1.3 (0.9-2.9) |
| Clearance (L/h) | 180 (1-652) | 32.3 (16-55) |
| In vitro Metabolism | ||
| Primary Pathway | Blood Esterases | Glucuronidation |
| Metabolite | DHA | α-DHA-β-glucuronide |
| Excretion | ||
| Urine | Unknown | Unknown |
PK=pharmacokinetics, AS=artesunate, DHA=dihydroartemisinin, Cmax=maximum concentration, AUC=area under the concentration-time curve
Specific Populations
Pediatric
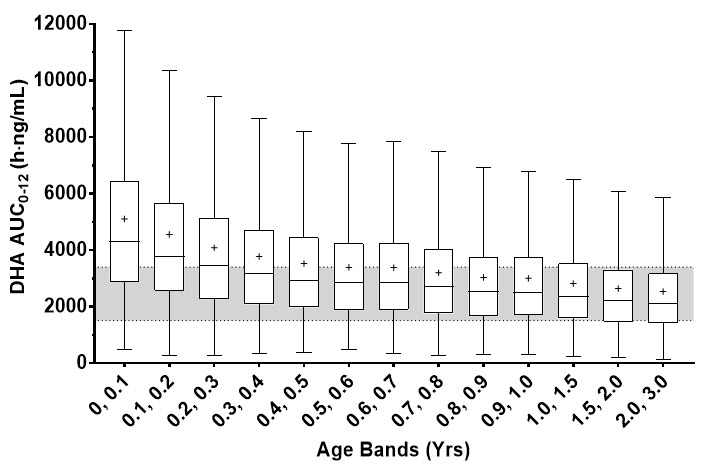
PK simulations, using a published population-based meta-analysis of DHA PK indicate a dosing regimen of 2.4 mg/kg results in comparable or greater predicted steady-state DHA AUC0-12 in infants less than 6 month of age compared to that observed in older children or adults (Figure 1). The difference in predicted exposures in infants less than 6 month of age is presumed to be due to immature development of the UGT elimination pathway for DHA.
Figure 1. Predicted Steady-State DHA AUC in Pediatric Patients 0 to 3 Years of Age with Severe Malaria after 2.4 mg/kg Artesunate for Injection:
Each box represents the 25th and 75th percentile of the DHA exposure measure. The bar and cross inside the box represent the median and mean respectively, whiskers represent 1.5 times the interquartile range. The gray band represents the interquartile range for patients weighing 20 to 25 kg (8 to 10 years of age) and adults. Clearance was estimated using a combination of allometric weight scaling with a sigmoid function to account for organ maturation.
Pregnant Women
In a published PK study involving 20 pregnant women with acute uncomplicated malaria administered 4 mg/kg IV artesunate, systemic exposures (Cmax and AUC) of artesunate and DHA were comparable between the pregnant acute malaria patients and the 3-months post-partum healthy patients. Therefore, no dose adjustment is necessary.
Published Clinical Drug-Drug Interaction Studies
Nevirapine
Co-administration of oral artesunate with nevirapine resulted in a decrease in Cmax and AUC of DHA by 59% and 68%, respectively. This reduction in systemic PK exposure of DHA is also likely to occur with Artesunate for Injection and may result in the potential loss of antimalarial efficacy.
Ritonavir
Co-administration of oral artesunate with ritonavir resulted in a 27% and 38% decrease in Cmax and AUC, respectively of DHA. This reduction in systemic PK exposure of DHA is also likely to occur with Artesunate for Injection and may result in the potential loss of antimalarial efficacy.
Other Anti-Malarial Drugs
No clinically significant drug interactions were reported with co-administration of oral artesunate with atovaquone/proguanil, mefloquine, amodiaquine and sulfadoxine/pyrimethamine. Therefore, drug interactions between Artesunate for Injection and these drugs are not expected.
In Vitro Studies
In vitro studies demonstrated that artesunate and DHA are not metabolized to any significant extent by CYP1A2, 2D6, 2C19, 2A6, 2E1 or 3A; therefore, no dosage adjustments are needed for inhibitors/inducers of these enzymes when co-administered with Artesunate for Injection. DHA is a substrate of UDP-UGT 1A9 or UGT 2B7.
Transporter Systems
DHA is not a substrate or inhibitor of P-gp or BCRP. Artesunate is a substrate of BCRP and P-gp. Artesunate is a weak inhibitor of OATP1B1 (IC50 = 19 mcg/mL) and OAT3.
12.4. Microbiology
Mechanism of Action
Artesunate is rapidly metabolized into an active metabolite DHA. Artesunate and DHA, like other artemisinins contain an endoperoxide bridge that is activated by heme iron leading to oxidative stress, inhibition of protein and nucleic acid synthesis, ultrastructural changes as well as a decrease in parasite growth and survival.
Both artesunate and DHA are active against the different asexual forms of the Plasmodium parasites and clear parasitemia within 48 to 72 hours.
Antimicrobial Activity
Artesunate and DHA are active against the blood-stage asexual parasites and gametocytes of Plasmodium species including the chloroquine resistant strains [see Clinical Studies (14)]. However, artesunate and DHA are not active against the hypnozoite liver stage forms of P. vivax and P. ovale.
Resistance
There is a potential for development of resistance to artesunate and DHA. Strains of P. falciparum with a decrease in sensitivity to artesunate can be selected in vitro or in vivo. Alterations in some genetic regions of P. falciparum genes such as multidrug resistant 1 (pfmdr1), chloroquine resistance transporter (pfcrt), and kelch13 (K13) have been reported based on in vitro testing and/or identification of isolates in endemic areas where artemisinin therapy has been used.
Recrudescence occurs in patients treated with artesunate monotherapy. Decreased sensitivity to artesunate and other artemisinins, manifesting clinically as slower rates of parasite clearance has been documented in some parts of Southeast Asia and is associated with mutation in the K13 gene.
Cross-resistance
Nonclinical studies in vitro and in malaria animal models suggest a potential for development of cross-resistance with quinine, halofantrine, and amodiaquine. However, the clinical significance of these findings is not known.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies have not been conducted with artesunate.
Mutagenesis
Artesunate was negative in an in vitro bacterial reverse mutation assay, an in vitro Chinese hamster ovary chromosome aberration assay, and an in vivo mouse bone marrow micronucleus assay when administered orally. However, the published literature indicates that artesunate induced DNA damage in human lymphocytes and Hep2G liver cells in a Comet assay and increased micronuclei formation in human lymphocytes. The published literature also indicates that in vivo, artesunate is positive for micronucleus formation but negative for DNA damage in peripheral blood cells in mice following oral administration. No in vivo genetic toxicology studies have been conducted with intravenously administered artesunate.
Fertility
Fertility studies in animals have not been conducted with intravenously administered artesunate.
No significant changes in reproductive organs (i.e., gross, microscopic or histologic lesions or organ weights) or sperm motility, counts or morphology were observed in rats and dogs following 28 days of repeated dosing with intravenously administered artesunate. However, in the published literature, rats and mice administered oral or intraperitoneal artesunate as a single dose or repeated dosing (3 days to 6 weeks) displayed histopathological changes of the seminiferous tubules and altered spermatogenesis (increased percentage of abnormal sperm and/or decreased sperm motility and viability) at doses ranging from approximately 0.2- to 1.3-times the clinical dose based on BSA comparisons. Given the conflicting findings, in the absence of fertility study(ies) conducted with intravenously administered artesunate, the clinical relevance of the animal data on human fertility is uncertain.
14. Clinical Studies
Overview
The efficacy of intravenous artesunate for the treatment of severe malaria was evaluated in a randomized active-controlled trial in Asia (Trial 1) and a supportive published randomized active-controlled trial in Africa (Trial 2).
Trial 1
Trial 1 was an international randomized, open-label, multicenter trial conducted in Bangladesh, India, Indonesia and Myanmar. Hospitalized patients with severe malaria were treated intravenously with either artesunate or quinine. Artesunate was administered at 2.4 mg/kg IV at 0, 12 and 24 hours and then every 24 hours until the patient could tolerate oral medication. Quinine was given IV at 20 mg/kg over 4 hours, followed by 10 mg/kg over 2 to 8 hours, 3 times daily until oral therapy could be started.
Trial 1 consisted of 1461 randomized patients, including 202 pediatric patients <15 years (14%). The median age was 28 years (range 2-87 years) and 74% were male. Malaria was confirmed on blood smear in 1382 patients (95%). Coma was present on admission in 588 patients (40%) and 229 patients (16%) had parasitemia >10%. Of the 1358 patients with hemoglobin measurement on admission, 94 patients (7%) had documented severe anemia (hemoglobin <5 g/dL).
Table 3 reports the in-hospital mortality results for Trial 1. The in-hospital mortality rate in the artesunate group (13%) was significantly lower than the rate in the quinine group (21%).
Table 3. In-hospital Mortality in Patients Treated for Severe Malaria in Trial 1, All Randomized Patients:
| Artesunate (N=730) | Quinine (N=731)2 | Odds Ratio (95% CI)1 | |
|---|---|---|---|
| In-hospital mortality | 96 (13%) | 150 (21%) | 0.59 (0.44, 0.79) |
1 The odds ratio and 95% CI (confidence interval) were calculated using the Cochran-Mantel-Haenszel approach adjusted by study site.
2 A single patient randomized to quinine arm did not receive any doses of the study drug.
Trial 2
Additional supportive evidence for efficacy was obtained from a published international, randomized, open-label, multicenter trial comparing parenteral artesunate to parenteral quinine in pediatric patients (<15 years of age) with severe malaria in nine African countries (Trial 2). Dosing was similar to Trial 1, except that both artesunate and quinine could be administered either intravenously or intramuscularly (not an approved route of administration). Compared to quinine, treatment with artesunate showed a similar advantage with respect to in-hospital mortality as in Trial 1.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.