YONSA Tablet Ref.[50142] Active ingredients: Abiraterone
Revision Year: 2022
Product description
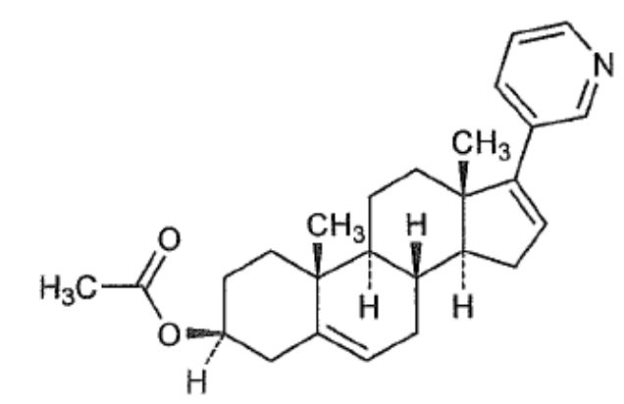
Abiraterone acetate, the active ingredient of YONSA tablet is the acetyl ester of abiraterone. Abiraterone is an inhibitor of CYP17 (17α-hydroxylase/C17,20-lyase). Each YONSA Tablet contains 125 mg of abiraterone acetate. Abiraterone acetate is designated chemically as (3β)17-(3-pyridinyl) androsta-5,16-dien-3-yl acetate and its structure is:
Abiraterone acetate is micronized (smaller particle size) white to off-white, non-hygroscopic, crystalline powder. Its molecular formula is C26H33NO2 and it has a molecular weight of 391.55. Abiraterone acetate is a lipophilic compound with an octanol-water partition coefficient of 5.12 (Log P) and is practically insoluble in water. The pKa of the aromatic nitrogen is 5.19.
Inactive ingredients in the tablets are lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, sodium stearyl fumarate, butylated hydroxyanisole, butylated hydroxytoluene.
| Dosage Forms and Strengths |
|---|
|
YONSA (abiraterone acetate) tablets, 125 mg, are white to off-white, oval-shaped tablets debossed with "125 FP" on one side. |
| How Supplied |
|---|
|
YONSA (abiraterone acetate) tablets, 125 mg: White to off-white, oval-shaped tablets debossed with "125 FP" on one side 120 tablets available in high-density polyethylene bottles with child resistant closure NDC Number 47335-401-81 Manufactured for: Sun Pharma Global FZE Distributed by: Sun Pharmaceutical Industries, Inc., Cranbury, NJ 08512 |
Drugs
| Drug | Countries | |
|---|---|---|
| YONSA | New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.