ABRAXANE Powder for suspension for infusion Ref.[7797] Active ingredients: Paclitaxel
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Celgene Europe B.V., Winthontlaan 6 N, 3526 KV Utrecht, Netherlands
Therapeutic indications
Abraxane monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated (see section 4.4).
Abraxane in combination with gemcitabine is indicated for the first-line treatment of adult patients with metastatic adenocarcinoma of the pancreas.
Abraxane in combination with carboplatin is indicated for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for potentially curative surgery and/or radiation therapy.
Posology and method of administration
Abraxane should only be administered under the supervision of a qualified oncologist in units specialised in the administration of cytotoxic agents. It should not be substituted for or with other paclitaxel formulations.
Posology
Breast cancer
The recommended dose of Abraxane is 260 mg/m² administered intravenously over 30 minutes every 3 weeks.
Dose adjustments during treatment of breast cancer
Patients who experience severe neutropenia (neutrophil count <500 cells/mm³ for a week or longer) or severe sensory neuropathy during Abraxane therapy should have the dose reduced to 220 mg/m² for subsequent courses. Following recurrence of severe neutropenia or severe sensory neuropathy, additional dose reduction should be made to 180 mg/m². Abraxane should not be administered until neutrophil counts recover to >1500 cells/mm³. For Grade 3 sensory neuropathy, withhold treatment until resolution to Grade 1 or 2, followed by a dose reduction for all subsequent courses.
Pancreatic adenocarcinoma
The recommended dose of Abraxane in combination with gemcitabine is 125 mg/m² administered intravenously over 30 minutes on Days 1, 8 and 15 of each 28-day cycle. The concurrent recommended dose of gemcitabine is 1000 mg/m² administered intravenously over 30 minutes immediately after the completion of Abraxane administration on Days 1, 8 and 15 of each 28-day cycle.
Dose adjustments during treatment of pancreatic adenocarcinoma
Table 1. Dose level reductions for patients with pancreatic adenocarcinoma:
| Dose Level | Abraxane Dose (mg/m²) | Gemcitabine Dose (mg/m²) |
|---|---|---|
| Full dose | 125 | 1000 |
| 1st dose level reduction | 100 | 800 |
| 2nd dose level reduction | 75 | 600 |
| If additional dose reduction required | Discontinue treatment | Discontinue treatment |
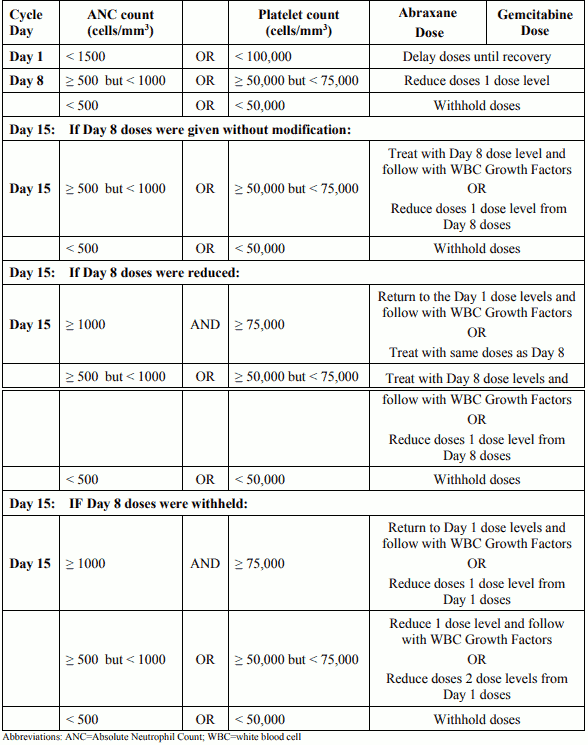
Table 2. Dose modifications for neutropenia and/or thrombocytopenia at the start of a cycle or within a cycle for patients with pancreatic adenocarcinoma:
Table 3. Dose modifications for other adverse drug reactions in patients with pancreatic adenocarcinoma:
| Adverse Drug Reaction (ADR) | Abraxane Dose | Gemcitabine Dose |
|---|---|---|
| Febrile Neutropenia: Grade 3 or 4 | Withhold doses until fever resolves and ANC ≥ 1500; resume at next lower dose levela | |
| Peripheral Neuropathy: Grade 3 or 4 | Withhold dose until improves to ≤ Grade 1; resume at next lower dose levela | Treat with same dose |
| Cutaneous Toxicity: Grade 2 or 3 | Reduce to next lower dose levela; discontinue treatment if ADR persists | |
| Gastrointestinal Toxicity: Grade 3 mucositis or diarrhoea | Withhold doses until improves to ≤ Grade 1; resume at next lower dose levela | |
a See Table 1 for dose level reductions
Non-small cell lung cancer
The recommended dose of Abraxane is 100 mg/m² administered as an intravenous infusion over 30 minutes on Days 1, 8 and 15 of each 21-day cycle. The recommended dose of carboplatin is AUC = 6 mg•min/mL on Day 1 only of each 21-day cycle, beginning immediately after the end of Abraxane administration.
Dose adjustments during treatment of non-small cell lung cancer
Abraxane should not be administered on Day 1 of a cycle until absolute neutrophil count (ANC) is ≥1500 cells/mm³ and platelet count is ≥100,000 cells/mm³. For each subsequent weekly dose of Abraxane, patients must have an ANC ≥500 cells/mm³ and platelets >50,000 cells/mm³ or the dose is to be withheld until counts recover. When counts recover, resume dosing the following week according to the criteria in Table 4. Reduce subsequent dose only if criteria in Table 4 are met.
Table 4. Dose reductions for haematologic toxicities in patients with non-small cell lung cancer:
| Haematologic Toxicity | Occurrence | Dose of Abraxane (mg/m²)1 | Dose of carboplatin (AUC mg•min/ml)1 |
|---|---|---|---|
| Nadir ANC <500/mm3 with neutropenic fever > 38°C OR Delay of next cycle due to persistent neutropenia2 (Nadir ANC <1.500/mm³) OR Nadir ANC <500/mm³ for >1 week | First | 75 | 4.5 |
| Second | 50 | 3.0 | |
| Third | Discontinue Treatment | ||
| Ναδίρ αιμοπεταλίων <50.000/mm³ | First | 75 | 4.5 |
| Second | Discontinue Treatment | ||
1 On Day 1 of the 21-day cycle reduce the dose of Abraxane and carboplatin simultaneously. On Days 8 or 15 of the 21-day cycle reduce the dose of Abraxane; reduce the dose of carboplatin in the subsequent cycle.
2 Maximum of 7 days post scheduled Day 1 dose of next cycle.
For Grade 2 or 3 cutaneous toxicity, Grade 3 diarrhoea, or Grade 3 mucositis, interrupt treatment until the toxicity improves to ≤ Grade 1, then restart treatment according to the guidelines in Table 5. For ≥ Grade 3 peripheral neuropathy, withhold treatment until resolution to ≤ Grade 1. Treatment may be resumed at the next lower dose level in subsequent cycles according to the guidelines in Table 5. For any other Grade 3 or 4 non-haematologic toxicity, interrupt treatment until the toxicity improves to ≤ Grade 2, then restart treatment according to the guidelines in Table 5.
Table 5. Dose reductions for non-haematologic toxicities in patients with non-small cell lung cancer:
| Non-haematologic Toxicity | Occurrence | Dose of Abraxane (mg/m²)1 | Dose of carboplatin (AUC mg•min/ml)1 |
|---|---|---|---|
| Grade 2 or 3 cutaneous toxicity. Grade 3 diarrhoea. Grade 3 mucositis. ≥ Grade 3 peripheral neuropathy. Any other Grade 3 or 4 nonhaematologic toxicity | First | 75 | 4.5 |
| Second | 50 | 3.0 | |
| Third | Discontinue Treatment | ||
| Grade 4 cutaneous toxicity, diarrhoea, or mucositis | First | Discontinue Treatment | |
1 On Day 1 of the 21-day cycle reduce the dose of Abraxane and carboplatin simultaneously. On Days 8 or 15 of the 21-day cycle reduce the dose of Abraxane; reduce the dose of carboplatin in the subsequent cycle.
Special populations
Patients with hepatic impairment
For patients with mild hepatic impairment (total bilirubin >1 to ≤1.5 x ULN and aspartate aminotransferase [AST] ≤ 10 x ULN), no dose adjustments are required, regardless of indication. Treat with same doses as patients with normal hepatic function.
For metastatic breast cancer patients and non-small cell lung cancer patients with moderate to severe hepatic impairment (total bilirubin >1.5 to ≤5 x ULN and AST ≤10 x ULN), a 20% reduction in dose is recommended. The reduced dose may be escalated to the dose for patients with normal hepatic function if the patient is tolerating the treatment for at least two cycles (see sections 4.4 and 5.2).
For patients with metastatic adenocarcinoma of the pancreas that have moderate to severe hepatic impairment, there are insufficient data to permit dosage recommendations (see sections 4.4 and 5.2).
For patients with total bilirubin >5 x ULN or AST >10 x ULN, there are insufficient data to permit dosage recommendations regardless of indication (see sections 4.4 and 5.2).
Patients with renal impairment
Adjustment of the starting Abraxane dose is not required for patients with mild to moderate renal impairment (estimated creatinine clearance ≥30 to <90 ml/min). There are insufficient data available to recommend dose modifications of Abraxane in patients with severe renal impairment or end stage renal disease (estimated creatinine clearance <30 ml/min) (see section 5.2).
Older people
No additional dosage reductions, other than those for all patients, are recommended for patients 65 years and older.
Of the 229 patients in the randomized study who received Abraxane monotherapy for breast cancer, 13% were at least 65 years of age and <2% were 75 years and older. No toxicities occurred notably more frequently among patients at least 65 years of age who received Abraxane. However, a subsequent analysis in 981 patients receiving Abraxane monotherapy for metastatic breast cancer, of which 15% were ≥65 years old and 2% were ≥75 years old, showed a higher incidence of epistaxis, diarrhoea, dehydration, fatigue and peripheral oedema in patients ≥ 65 years.
Of the 421 patients with pancreatic adenocarcinoma in the randomized study who received Abraxane in combination with gemcitabine, 41% were 65 years and older and 10% were 75 years and older. In patients aged 75 years and older who received Abraxane and gemcitabine, there was a higher incidence of serious adverse reactions and adverse reactions that led to treatment discontinuation (see section 4.4). Patients with pancreatic adenocarcinoma aged 75 years and older should be carefully assessed before treatment is considered (see section 4.4).
Of the 514 patients with non-small cell lung cancer in the randomized study who received Abraxane in combination with carboplatin, 31% were 65 years or older and 3.5% were 75 years or older. Myelosuppression events, peripheral neuropathy events, and arthralgia were more frequent in patients 65 years or older compared to patients younger than 65 years of age. There is limited experience of Abraxane/carboplatin use in patients 75 years or older.
Pharmacokinetic/pharmacodynamic modelling using data from 125 patients with advanced solid tumours indicates that patients ≥65 years of age may be more susceptible to development of neutropenia within the first treatment cycle.
Paediatric population
The safety and efficacy of Abraxane in children and adolescents aged 0-17 years has not been established. There is no relevant use of Abraxane in the paediatric population in the indication of metastatic breast cancer or pancreatic adenocarcinoma or non-small cell lung cancer.
Method of administration
Administer reconstituted Abraxane suspension intravenously using an infusion set incorporating a 15 µm filter. Following administration, it is recommended that the intravenous line be flushed with sodium chloride 9 mg/ml (0.9%) solution for injection to ensure administration of the complete dose.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
There is no known antidote for paclitaxel overdose. In the event of an overdose, the patient should be closely monitored. Treatment should be directed at the major anticipated toxicities, which are bone marrow suppression, mucositis and peripheral neuropathy.
Shelf life
Unopened vials: 3 years.
Stability of reconstituted suspension in the vial: Chemical and physical in-use stability has been demonstrated for 24 hours at 2°C-8°C in the original carton, protected from light.
Stability of the reconstituted suspension in the infusion bag: Chemical and physical in-use stability has been demonstrated for 24 hours at 2°C-8°C followed by 4 hours at 25°C, protected from light.
However, from a microbiological point of view, unless the method of reconstituting and filling of the infusion bags precludes the risks of microbial contamination, the product should be used immediately after reconstitution and filling of the infusion bags.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
The total combined storage time of reconstituted medicinal product in the vial and in the infusion bag when refrigerated and protected from light is 24 hours. This may be followed by storage in the infusion bag for 4 hours below 25°C.
Special precautions for storage
Unopened vials
Keep the vial in the outer carton in order to protect from light. Neither freezing nor refrigeration adversely affects the stability of the product. This medicinal product does not require any special temperature storage conditions.
Reconstituted suspension
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
50 ml vial (type 1 glass) with a stopper (butyl rubber), with an overseal (aluminium), containing 100 mg of paclitaxel formulated as albumin bound nanoparticles.
100 ml vial (type 1 glass) with a stopper (butyl rubber), with an overseal (aluminium), containing 250 mg of paclitaxel formulated as albumin bound nanoparticles.
Pack size of one vial.
Special precautions for disposal and other handling
Preparation and administration precautions
Paclitaxel is a cytotoxic anticancer medicinal product and, as with other potentially toxic compounds, caution should be exercised in handling Abraxane. The use of gloves, goggles and protective clothing is recommended. If the suspension contacts the skin, the skin should be washed immediately and thoroughly with soap and water. If it contacts mucous membranes, the membranes should be flushed thoroughly with water. Abraxane should only be prepared and administered by personnel appropriately trained in the handling of cytotoxic agents. Pregnant staff should not handle Abraxane.
Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible infiltration during administration of the medicinal product. Limiting the infusion of Abraxane to 30 minutes, as directed, reduces the likelihood of infusion-related reactions.
Reconstitution and administration of the product
Abraxane is supplied as a sterile lyophilised powder for reconstitution before use. After reconstitution, each ml of suspension contains 5 mg of paclitaxel formulated as albumin bound nanoparticles.
100 mg vial: Using a sterile syringe, 20 ml of sodium chloride 9 mg/ml (0.9%) solution for infusion should slowly be injected into a vial of Abraxane over a minimum of 1 minute.
250 mg vial: Using a sterile syringe, 50 ml of sodium chloride 9 mg/ml (0.9%) solution for infusion should slowly be injected into a vial of Abraxane over a minimum of 1 minute.
The solution should be directed onto the inside wall of the vial. The solution should not be injected directly onto the powder as this will result in foaming.
Once the addition is complete, the vial should be allowed to stand for a minimum of 5 minutes to ensure proper wetting of the solid. Then, the vial should gently and slowly be swirled and/or inverted for at least 2 minutes until complete resuspension of any powder occurs. The generation of foam must be avoided. If foaming or clumping occurs, the solution must stand for at least 15 minutes until foam subsides.
The reconstituted suspension should be milky and homogenous without visible precipitates. Some settling of the reconstituted suspension may occur. If precipitates or settling are visible, the vial should be gently inverted again to ensure complete resuspension prior to use.
Inspect the suspension in the vial for particulate matter. Do not administer the reconstituted suspension if particulate matter is observed in the vial.
The exact total dosing volume of 5 mg/ml suspension required for the patient should be calculated and the appropriate amount of reconstituted Abraxane should be injected into an empty, sterile, PVC or non-PVC type intravenous bag.
The use of medical devices containing silicone oil as a lubricant (i.e. syringes and IV bags) to reconstitute and administer Abraxane may result in the formation of proteinaceous strands. Administer Abraxane using an infusion set incorporating a 15 µm filter to avoid administration of these strands. Use of a 15 µm filter removes strands and does not change the physical or chemical properties of the reconstituted product. Use of filters with a pore size less than 15 µm may result in blockage of the filter.
The use of specialized di(2-ethylhexyl)phthalate (DEHP)-free solution containers or administration sets is not necessary to prepare or administer Abraxane infusions.
Following administration, it is recommended that the intravenous line be flushed with sodium chloride 9 mg/ml (0.9%) solution for injection to ensure administration of the complete dose.
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.