ABSTRAL Sublingual tablet Ref.[7245] Active ingredients: Fentanyl
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Kyowa Kirin Ltd, Galabank Business Park, Galashiels, TD1 1QH, UK
Therapeutic indications
Management of breakthrough pain in adult patients using opioid therapy for chronic cancer pain. Breakthrough pain is a transient exacerbation of otherwise controlled chronic background pain.
Posology and method of administration
Abstral should only be administered to patients who are considered tolerant to their opioid therapy for persistent cancer pain. Patients can be considered opioid tolerant if they take at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
Method of administration
Abstral sublingual tablets should be administered directly under the tongue at the deepest part. Abstral sublingual tablets should not be swallowed, but allowed to completely dissolve in the sublingual cavity without chewing or sucking. Patients should be advised not to eat or drink anything until the sublingual tablet is completely dissolved.
In patients who have a dry mouth water may be used to moisten the buccal mucosa before taking Abstral.
Dose titration
The object of dose titration is to identify an optimal maintenance dose for ongoing treatment of breakthrough pain episodes. This optimal dose should provide adequate analgesia with an acceptable level of adverse reactions.
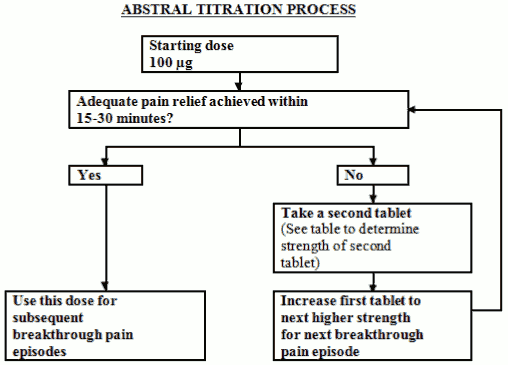
The optimal dose of Abstral will be determined by upward titration, on an individual patient basis. Several doses are available for use during the dose titration phase. The initial dose of Abstral used should be 100 micrograms, titrating upwards as necessary through the range of available dosage strengths.
Patients should be carefully monitored until an optimal dose is reached.
Switching from other fentanyl containing products to Abstral must not occur at a 1:1 ratio because of different absorption profiles. If patients are switched from another fentanyl containing product, a new dose titration with Abstral is required.
The following dose regimen is recommended for titration, although in all cases the physician should take into account the clinical need of the patient, age and concomitant illness.
All patients must start therapy with a single 100 microgram sublingual tablet. If adequate analgesia is not obtained within 15-30 minutes of administration of a single sublingual tablet, a supplemental (second) 100 microgram sublingual tablet may be administered. If adequate analgesia is not obtained within 15-30 minutes of the first dose an increase in dose to the next highest tablet strength should be considered for the next episode of breakthrough pain (Refer to figure below).
Dose escalation should continue in a stepwise manner until adequate analgesia with tolerable adverse reactions is achieved. The dose strength for the supplemental (second) sublingual tablet should be increased from 100 to 200 micrograms at doses of 400 micrograms and higher. This is illustrated in the schedule below. No more than two (2) doses should be administered for a single episode of breakthrough pain during this titration phase.
| Strength (micrograms) of first sublingual tablet per episode of breakthrough pain | Strength (micrograms) of supplemental (second) sublingual tablet to be taken 15-30 minutes after first tablet, if required |
|---|---|
| 100 | 100 |
| 200 | 100 |
| 300 | 100 |
| 400 | 200 |
| 600 | 200 |
| 800 | - |
If adequate analgesia is achieved at the higher dose, but undesirable effects are considered unacceptable, an intermediate dose (using the 100 microgram sublingual tablet where appropriate) may be administered.
During titration, patients can be instructed to use multiples of 100 microgram tablets and/or 200 microgram tablets for any single dose. No more than four (4) tablets should be used at any one time.
The efficacy and safety of doses higher than 800 micrograms have not been evaluated in clinical studies in patients.
In order to minimise the risk of opioid–related adverse reactions and to identify the appropriate dose, it is imperative that patients be monitored closely by health professionals during the titration process.
During titration patients should wait at least 2 hours before treating another episode of breakthrough pain with Abstral.
Maintenance therapy
Once an appropriate dose has been established, which may be more than one tablet, patients should be maintained on this dose and should limit consumption to a maximum of four Abstral doses per day.
During the maintenance period patients should wait at least 2 hours before treating another episode of breakthrough pain with Abstral.
Dose re-adjustment
If the response (analgesia or adverse reactions) to the titrated Abstral dose markedly changes, an adjustment of dose may be necessary to ensure that an optimal dose is maintained.
If more than four episodes of breakthrough pain are experienced per day over a period of more than four consecutive days, then the dose of the long acting opioid used for persistent pain should be re-evaluated. If the long acting opioid or dose of long acting opioid is changed the Abstral dose should be re-evaluated and re-titrated as necessary to ensure the patient is on an optimal dose.
It is imperative that any dose re-titration of any analgesic is monitored by a health professional.
In absence of adequate pain control, the possibility of hyperalgesia, tolerance and progression of underlying disease should be considered (see section 4.4).
Discontinuation of therapy
Abstral should be discontinued immediately if the patient no longer experiences breakthrough pain episodes. The treatment for the persistent background pain should be kept as prescribed.
If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor in order to avoid the possibility of abrupt withdrawal effects.
Use in children and adolescents
Abstral must not be used in patients less than 18 years of age due to a lack of data on safety and efficacy.
Use in older people
Dose titration needs to be approached with particular care and patients observed carefully for signs of fentanyl toxicity (see section 4.4).
Use in patients with renal and hepatic impairment
Patients with kidney or liver dysfunction should be carefully observed for signs of fentanyl toxicity during the Abstral titration phase (see section 4.4).
Overdose
The symptoms of fentanyl overdose are an extension of its pharmacological actions, the most serious effect being respiratory depression, which may lead to respiratory arrest. Coma is also known to occur.
Management of opioid overdose in the immediate term includes removal of any remaining Abstral sublingual tablets from the mouth, physical and verbal stimulation of the patient and an assessment of the level of consciousness. A patent airway should be established and maintained. If necessary an oropharyngeal airway or endotracheal tube should be inserted, oxygen administered and mechanical ventilation initiated, as appropriate. Adequate body temperature and parenteral fluid intake should be maintained.
For the treatment of accidental overdose in opioid-naïve individuals, naloxone or other opioid antagonists should be used as clinically indicated and in accordance with their Summary of Product Characteristics. Repeated administration of the opioid antagonist may be necessary if the duration of respiratory depression is prolonged.
Care should be taken when using naloxone or other opioid antagonists to treat overdose in opioid-maintained patients, due to the risk of precipitating an acute withdrawal syndrome.
If severe or persistent hypotension occurs, hypovolaemia should be considered, and the condition should be managed with appropriate parenteral fluid therapy.
Muscle rigidity interfering with respiration has been reported with fentanyl and other opioids. In this situation, endotracheal intubation, assisted ventilation and administration of opioid antagonists as well as muscle relaxants may be requested.
Shelf life
2 years.
Special precautions for storage
Do not store above 25°C.
Store in the original blister package in order to protect from moisture.
Nature and contents of container
Abstral sublingual tablets are packaged in child resistant blisters of OPA/Aluminium/PVC pockets with paper/polyester/Aluminium lidding contained in a cardboard outer carton. The packaging is colour-coded for each Abstral sublingual tablet strength.
Pack size: Packs of 10 or 30 sublingual tablets. Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Waste material should be disposed of safely. Patients/carers should be encouraged to return any unused product to the Pharmacy, where it should be disposed of in accordance with national and local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.