ACTILYSE Powder and solvent for solution Ref.[6149] Active ingredients: Alteplase
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Boehringer Ingelheim Limited, Ellesfield Avenue, Bracknell, Berkshire RG12 8YS
Therapeutic indications
Thrombolytic treatment in acute myocardial infarction
- 90 minutes (accelerated) dose regimen (see section 4.2): for patients in whom treatment can be started within 6 hours after symptom onset
- 3 hour dose regimen (see section 4.2): for patients in whom treatment can be started between 6-12 hours after symptom onset provided that the diagnosis has been clearly confirmed.
Actilyse has proven to reduce 30-day-mortality in patients with acute myocardial infarction.
Thrombolytic treatment in acute massive pulmonary embolism with haemodynamic instability
The diagnosis should be confirmed whenever possible by objective means such as pulmonary angiography or non-invasive procedures such as lung scanning. There is no evidence for positive effects on mortality and late morbidity related to pulmonary embolism.
Fibrinolytic treatment of acute ischaemic stroke
Treatment must be started as early as possible within 4.5 hours after onset of stroke symptoms and after exclusion of intracranial haemorrhage by appropriate imaging techniques (e.g. cranial computerised tomography or other diagnostic imaging method sensitive for the presence of haemorrhage). The treatment effect is time-dependent; therefore earlier treatment increases the probability of a favourable outcome.
Posology and method of administration
Actilyse should be given as early as possible after symptom onset. The following dose guidelines apply.
Acute myocardial infarction
Posology
a) 90 minutes (accelerated) dose regimen for patients with acute myocardial infarction, in whom treatment can be started within 6 hours after symptom onset.
In patients with a body weight ≥65 kg:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 15 mg as an intravenous bolus, immediately followed by | 15 ml | 7.5 ml |
| 50 mg as an intravenous constant rate infusion over the first 30 minutes, immediately followed by | 50 ml | 25 ml |
| 35 mg as an intravenous constant rate infusion over 60 minutes, until the maximum total dose of 100 mg | 35 ml | 17.5 ml |
In patients with a body weight <65 kg the total dose should be weight adjusted according to the following table:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 15 mg as an intravenous bolus, immediately followed by | 15 ml | 7.5 ml |
| 0.75 mg/kg body weight (bw) as an intravenous constant rate infusion over the first 30 minutes, immediately followed by | 0.75 ml/kg bw | 0.375 ml/kg bw |
| 0.5 mg/kg body weight (bw) as an intravenous constant rate infusion over 60 minutes | 0.5 ml/kg bw | 0.25 ml/kg bw |
b) 3 h dose regimen for patients with acute myocardial infarction, in whom treatment can be started between 6 and 12 hours after symptom onset.
In patients with a body weight ≥65 kg:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 10 mg as an intravenous bolus, immediately followed by | 10 ml | 5 ml |
| 50 mg as an intravenous constant rate infusion over the first hour, immediately followed by | 50 ml | 25 ml |
| 40 mg as an intravenous constant rate infusion over 2 hours, until the maximum total dose of 100 mg | 40 ml | 20 ml |
In patients with a body weight <65 kg:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 10 mg as an intravenous bolus, immediately followed by | 10 ml | 5 ml |
| an intravenous constant rate infusion over 3 hours up to a maximum total dose of 1.5 mg/kg bw | 1.5 ml/kg bw | 0.75 ml/kg bw |
Adjunctive therapy: Antithrombotic adjunctive therapy is recommended according to the current international guidelines for the management of patients with ST-elevation myocardial infarction.
Method of administration
The reconstituted solution should be administered intravenously and is for immediate use.
2 mg vials of alteplase are not indicated for use in this indication. For instructions prior to reconstitution/administration, see section 6.6.
Acute massive pulmonary embolism
Posology
In patients with a body weight ≥65kg:
A total dose of 100 mg of alteplase should be administered in 2 hours. Most experience is available with the following dose regimen:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 10 mg as an intravenous bolus over 1-2 minutes, immediately followed by | 10 ml | 5 ml |
| 90 mg as an intravenous constant rate infusion over 2 hours until the maximum total dose of 100 mg | 90 ml | 45 ml |
In patients with a body weight <65 kg:
| Volume to be administered according to alteplase concentration | ||
|---|---|---|
| 1 mg/ml | 2 mg/ml | |
| 10 mg as an intravenous bolus over 1-2 minutes, immediately followed by | 10 ml | 5 ml |
| an intravenous constant rate infusion over 2 hours up to a maximum total dose of 1.5 mg/kg bw | 1.5 ml/kg bw | 0.75 ml/kg bw |
Adjunctive therapy: After treatment with Actilyse heparin therapy should be initiated (or resumed) when aPTT values are less than twice the upper limit of normal. The infusion should be adjusted to maintain aPTT between 50-70 seconds (1.5 to 2.5 fold of the reference value).
Method of administration
The reconstituted solution should be administered intravenously and is for immediate use.
2 mg vials of alteplase are not indicated for use in this indication. For instructions prior to reconstitution/administration, see section 6.6.
Acute ischaemic stroke
Treatment must only be performed under the responsibility and follow-up of a physician trained and experienced in neurovascular care, see sections 4.3 and 4.4.
Treatment with Actilyse must be started as early as possible within 4.5 hours of the onset of symptoms (see section 4.4). Beyond 4.5 hours after onset of stroke symptoms there is a negative benefit risk ratio associated with Actilyse administration and so it should not be administered (see section 5.1).
Posology
The recommended total dose is 0.9 mg alteplase/kg body weight (maximum of 90 mg) starting with 10% of the total dose as an initial intravenous bolus, immediately followed by the remainder of the total dose infused intravenously over 60 minutes.
DOSING TABLE FOR ACUTE ISCHAEMIC STROKE:
| By using the recommended standard concentration of 1 mg/ml the volume (ml) to be administered is equal to the recommended dosing value (mg) | |||
|---|---|---|---|
| Weight (kg) | Total Dose (mg) | Bolus Dose (mg) | Infusion Dose* (mg) |
| 40 | 36.0 | 3.6 | 32.4 |
| 42 | 37.8 | 3.8 | 34.0 |
| 44 | 39.6 | 4.0 | 35.6 |
| 46 | 41.4 | 4.1 | 37.3 |
| 48 | 43.2 | 4.3 | 38.9 |
| 50 | 45.0 | 4.5 | 40.5 |
| 52 | 46.8 | 4.7 | 42.1 |
| 54 | 48.6 | 4.9 | 43.7 |
| 56 | 50.4 | 5.0 | 45.4 |
| 58 | 52.2 | 5.2 | 47.0 |
| 60 | 54.0 | 5.4 | 48.6 |
| 62 | 55.8 | 5.6 | 50.2 |
| 64 | 57.6 | 5.8 | 51.8 |
| 66 | 59.4 | 5.9 | 53.5 |
| 68 | 61.2 | 6.1 | 55.1 |
| 70 | 63.0 | 6.3 | 56.7 |
| 72 | 64.8 | 6.5 | 58.3 |
| 74 | 66.6 | 6.7 | 59.9 |
| 76 | 68.4 | 6.8 | 61.6 |
| 78 | 70.2 | 7.0 | 63.2 |
| 80 | 72.0 | 7.2 | 64.8 |
| 82 | 73.8 | 7.4 | 66.4 |
| 84 | 75.6 | 7.6 | 68.0 |
| 86 | 77.4 | 7.7 | 69.7 |
| 88 | 79.2 | 7.9 | 71.3 |
| 90 | 81.0 | 8.1 | 72.9 |
| 92 | 82.8 | 8.3 | 74.5 |
| 94 | 84.6 | 8.5 | 76.1 |
| 96 | 86.4 | 8.6 | 77.8 |
| 98 | 88.2 | 8.8 | 79.4 |
| 100+ | 90.0 | 9.0 | 81.0 |
* given in a concentration of 1mg/mL over 60 min as a constant rate infusion.
Adjunctive therapy: The safety and efficacy of this regimen with concomitant administration of heparin or platelet aggregation inhibitors such as acetylsalicylic acid within the first 24 hours of onset of the symptoms have not been sufficiently investigated. Therefore, administration of intravenous heparin or platelet aggregation inhibitors such as acetylsalicylic acid should be avoided in the first 24 hours after treatment with Actilyse due to an increased haemorrhagic risk. If heparin is required for other indications (e.g. prevention of deep vein thrombosis) the dose should not exceed 10,000 IU per day, administered subcutaneously.
Method of administration
The reconstituted solution should be administered intravenously and is for immediate use.
2 mg vials of alteplase are not indicated for use in this indication. For instructions prior to reconstitution/administration, see section 6.6.
Paediatric population
There is limited experience with the use of Actilyse in children and adolescents. Actilyse is contraindicated for the treatment of acute ischaemic stroke in children and adolescents under 16 years of age (see section 4.3). The dose for adolescents 16-17 years old is the same as for adults (see section 4.4 for recommendations on prior imaging techniques to be used).
Overdose
Symptoms
If the maximum recommended dose is exceeded the risk of intracranial bleeding increases.
The relative fibrin specificity notwithstanding, a clinically significant reduction in fibrinogen and other blood coagulation components may occur after overdosage.
Therapy
In most cases, it is sufficient to await the physiological regeneration of these factors after the Actilyse therapy has been terminated. If, however, severe bleeding results, the infusion of fresh frozen plasma is recommended and if necessary, synthetic antifibrinolytics may be administered.
Shelf life
Unopened vials: 3 years.
Reconstituted solution: The reconstituted solution has been demonstrated to be stable for 24 hours at 2°C–8°C and for 8 hours at 25°C.
From a microbiological point of view, the product should be used immediately after reconstitution.
If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C.
Special precautions for storage
Store in the original package in order to protect from light.
Do not store above 25°C.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
Powder: 10 ml, 20 ml or 50 ml sterilised glass vials, sealed with sterile siliconised grey butyl-type stoppers with aluminium/plastic flip-off caps.
Solvent: For the 10 mg, 20 mg and 50 mg presentations, the water for injections is filled into either 10 ml, 20 ml or 50 ml vials, depending on the size of the powder vials. The water for injections vials are sealed with rubber stoppers and aluminium/plastic flip-off caps.
Transfer cannulas (included with presentations of 20 mg and 50 mg only)
Presentations:
10 mg:
1 vial with 467 mg powder for solution for injection and infusion
1 vial with 10 ml of water for injections
20 mg:
1 vial with 933 mg powder for solution for injection and infusion
1 vial with 20 ml of water for injections
1 transfer cannula
50 mg:
1 vial with 2333 mg powder for solution for injection and infusion
1 vial with 50 ml of water for injections
1 transfer cannula
Not all presentations may be marketed.
Special precautions for disposal and other handling
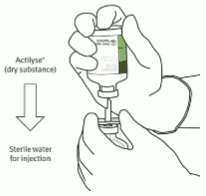
For reconstitution to a final concentration of 1 mg alteplase per ml the full volume of solvent provided should be transferred to the vial containing the Actilyse powder. To this purpose a transfer cannula is included with the 20 mg and 50 mg presentations, which is to be used. For the 10 mg presentation a syringe should be used.
For reconstitution to a final concentration of 2 mg alteplase per ml only half of the solvent provided should be used (as per table below). In these cases always a syringe should be used to transfer the required amount of solvent to the vial containing the Actilyse powder.
Under aseptic conditions the content of an injection vial of Actilyse (10 mg or 20 mg or 50 mg) is dissolved with water for injections according to the following table to obtain either a final concentration of 1 mg alteplase/ml or 2 mg alteplase/ml:
| Actilyse dry substance | 10 mg | 20 mg | 50 mg |
| (a) Volume of sterilised water for injections to be added to dry substance | 10 mL | 20 mL | 50 mL |
| Final concentration: | 1 mg alteplase/mL | 1 mg alteplase/mL | 1 mg alteplase/mL |
| (b) Volume of sterilised water for injections to be added to dry substance | 5 mL | 10 mL | 25 mL |
| Final concentration: | 2 mg alteplase/mL | 2 mg alteplase/mL | 2 mg alteplase/mL |
The reconstituted solution should then be administered intravenously. The 1 mg/mL reconstituted solution may be diluted further with sterile sodium chloride 9 mg/ml (0.9 %) solution for injection up to a minimal concentration of 0.2 mg/ml since the occurrence of turbidity of the reconstituted solution cannot be excluded. A further dilution of the 1 mg/mL reconstituted solution with sterilised water for injections or in general, the use of carbohydrate infusion solutions, e.g. dextrose is not recommended due to increasing formation of turbidity of the reconstituted solution. Actilyse should not be mixed with other medicinal products in the same infusion-vial (not even with heparin).
For incompatibilities see section 6.2.
The reconstituted solution is for single use only. Any unused solution or waste material should be disposed in accordance with the local requirements.
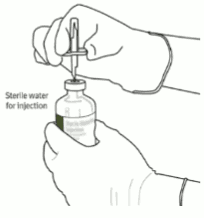
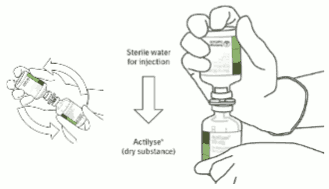
Instructions for reconstituting Actilyse:
1. Reconstitute immediately before administration.
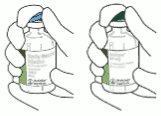
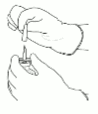
2. Remove the protective cap on the two vials containing the sterile water and Actilyse dry substance by flipping them up with a thumb.
3. Swab the rubber top of each vial with an alcohol wipe.
4. Remove the transfer cannula* from its cover. Do not disinfect or sterilize the transfer cannula; it is sterile. Take one cap off.
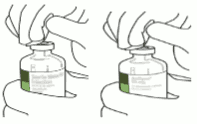
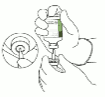
5. Stand the sterile water vial upright on a stable surface. From directly above, puncture the rubber stopper vertically in the stopper center with the transfer cannula, by pressing gently but firmly, without twisting.
6. Hold the sterile water vial and the transfer cannula steady with one hand using the two side flaps. Remove the remaining cap on top of the transfer cannula.
7. Hold the sterile water vial and the transfer cannula steady with one hand using the two side flaps. Hold the vial with Actilyse dry substance above the transfer cannula and position the tip of the transfer cannula right in the center of the stopper.
Push down the vial with the dry substance onto the transfer cannula from directly above, puncturing the rubber stopper vertically and gently but firmly without twisting.
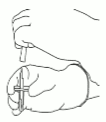
8. Invert the two vials and allow the water to drain completely into the dry substance.
9. Remove the empty water vial together with the transfer cannula. They can be disposed of.
10. Take the vial with reconstituted Actilyse and swirl gently to dissolve any remaining powder, but do not shake, as this will produce foam.
If there are bubbles, let the solution stand undisturbed for a few minutes to allow them to disappear.
11. The solution consists of 1mg/mL Actilyse. It should be clear and colourless to pale yellow and it should not contain any particles.
12. Remove the amount required using a needle and a syringe. Do not use the puncture location from the transfer cannula to avoid leakage.
13. Use immediately. Dispose of any unused solution.
(*if a transfer cannula is included in the kit. The reconstitution can also be performed with a syringe and a needle.)
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.