ADMELOG Solution for injection Ref.[10741] Active ingredients: Insulin lispro
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
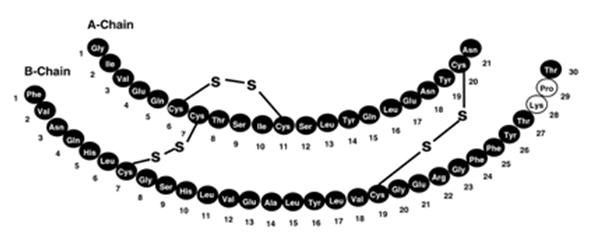
ADMELOG (insulin lispro injection) is a rapid-acting human insulin analog used to lower blood glucose. Insulin lispro is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli. Insulin lispro differs from human insulin in that the amino acid proline at position B28 is replaced by lysine and the lysine in position B29 is replaced by proline. Chemically, it is Lys(B28), Pro(B29) human insulin analog and has the empirical formula C257H383N65O77S6 and a molecular weight of 5808, both identical to that of human insulin.
ADMELOG has the following primary structure:
ADMELOG is a sterile, aqueous, clear, and colorless solution. Each milliliter of ADMELOG contains insulin lispro 100 units, 16 mg glycerin, 1.88 mg dibasic sodium phosphate, 3.15 mg metacresol, zinc oxide content adjusted to provide 0.0197 mg zinc ion, and Water for Injection. Insulin lispro has a pH of 7.0 to 7.8. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and/or sodium hydroxide.
| Dosage Forms and Strengths |
|---|
|
Insulin lispro injection 100 units per mL (U-100) is available as:
|
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ADMELOG: Insulin Lispro Injection 100 units per mL (U-100) is available as:
Each prefilled SoloStar pen is for use by a single patient. ADMELOG SoloStar pen must never be shared between patients, even if the needle is changed. Patients using ADMELOG vials must never share needles or syringes with another person. The ADMELOG SoloStar prefilled pen dials in 1-unit increments. sanofi-aventis U.S. LLC, Bridgewater, NJ 08807, A SANOFI COMPANY |
Drugs
| Drug | Countries | |
|---|---|---|
| ADMELOG | Canada, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.