ADMELOG Solution for injection Ref.[10741] Active ingredients: Insulin lispro
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Regulation of glucose metabolism is the primary activity of insulins and insulin analogs, including insulin lispro products. Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis and enhance protein synthesis.
12.2. Pharmacodynamics
Subcutaneous Administration
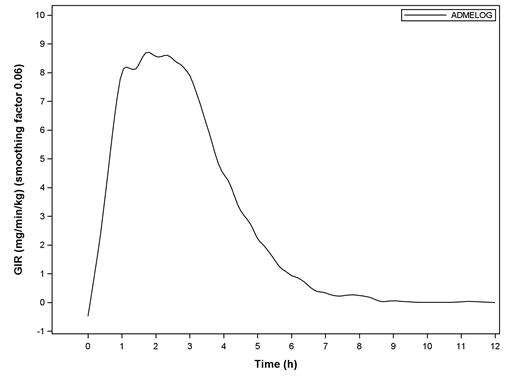
The pharmacodynamic profile of a single 0.3 unit/kg dose of ADMELOG administered subcutaneously was evaluated in a euglycemic clamp study enrolling 30 patients with type 1 diabetes. In this study, the mean (SD) time to maximum effect of ADMELOG (measured by the peak rate of glucose infusion) was approximately 2.07 (0.78) hours. The mean (SD) area under the glucose infusion rate curves (measure of overall pharmacodynamic effect) and mean (SD) maximum glucose infusion rate were 1953.5 (547.3) mg/kg and 9.97 (2.37) mg/min/kg, respectively (see Figure 1).
Figure 1. Mean Smoothed Glucose Infusion Rate?footnote? after Subcutaneous Injection of ADMELOG (0.3 unit/kg) in Patients with Type 1 Diabetes
* Body Weight Standardized
The time course of action of insulin and insulin analogs, including insulin lispro products, may vary considerably in different individuals or within the same individual. The rate of insulin absorption and, consequently, the onset of activity are known to be affected by the site of injection, exercise, and other variables [see Warnings and Precautions (5.2)].
Intravenous Administration
The glucose lowering effect of intravenous administration of another insulin lispro product, 100 units/mL, was tested in 21 patients with type 1 diabetes. For the study, the patients' usual doses of insulin were held, and blood glucose concentrations were allowed to reach a stable range of 200 to 260 mg/dL during a one to three-hour run-in phase. The run-in phase was followed by a 6-hour assessment phase. During the assessment phase, patients received intravenous infusion of another insulin lispro product, 100 units/mL, at an initial infusion rate of 0.5 units/hour. The infusion rate could be adjusted at regular timed intervals to achieve and maintain blood glucose concentrations between 100 to 160 mg/dL.
The mean blood glucose levels during the assessment phase for patients on another insulin lispro product, 100 units/mL, therapy are summarized below in Table 2. All patients achieved the targeted glucose range at some point during the 6-hour assessment phase. At the endpoint, blood glucose was within the target range (100 to 160 mg/dL) for 17 of 20 patients treated with another insulin lispro product, 100 units/mL. The average time (±SE) required to attain near normoglycemia was 129 ± 14 minutes for another insulin lispro product, 100 units/mL.
Table 2. Mean Blood Glucose Concentrations (mg/dL) During Intravenous Infusions of Another Insulin Lispro Product, 100 units/mL:
| Time from Start of Infusion (minutes) | Mean Blood Glucose (mg/dL) Intravenous* |
|---|---|
| 0 | 224 ± 16 |
| 30 | 205 ± 21 |
| 60 | 195 ± 20 |
| 120 | 165 ± 26 |
| 180 | 140 ± 26 |
| 240 | 123 ± 20 |
| 300 | 120 ± 27 |
| 360 | 122 ± 25 |
* Results shown as mean ± SD.
12.3. Pharmacokinetics
Absorption
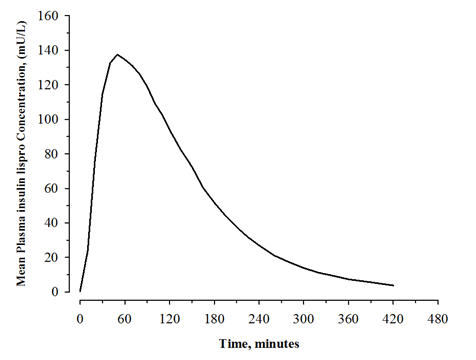
The pharmacokinetic profile of a single 0.3 unit/kg dose of ADMELOG administered subcutaneously was evaluated in a study enrolling 30 patients with type 1 diabetes. In this study, the mean observed area under the plasma insulin lispro concentration-time curve from time zero to infinity and peak plasma insulin lispro concentration were 12800 pg∙hr/mL and 5070 pg/mL, respectively. The median time to maximum plasma insulin lispro concentration was 0.83 hours after injection (see Figure 2).
Figure 2. Mean Plasma Concentrations of ADMELOG after a Single Subcutaneous Administration of ADMELOG (0.3 unit/kg) in Patients with Type 1 Diabetes:
The absolute bioavailability of another insulin lispro product, 100 units/mL, after subcutaneous injection ranges from 55% to 77% with doses between 0.1 to 0.2 unit/kg, inclusive.
Distribution
When administered intravenously as bolus injections of 0.1 and 0.2 unit/kg dose in two separate groups of healthy subjects, the mean volume of distribution of another insulin lispro product, 100 units/mL, appeared to decrease with increase in dose (1.55 and 0.72 L/kg, respectively).
Elimination
Metabolism
Human metabolism studies have not been conducted. However, animal studies indicate that the metabolism of another insulin lispro product, 100 units/mL, is identical to that of regular human insulin.
Excretion
When administered intravenously, another insulin lispro product, 100 units/mL demonstrated dose-dependent clearance, with a mean clearance of 21.0 mL/min/kg (0.1 unit/kg dose), and 9.6 mL/min/kg (0.2 unit/kg dose). Another insulin lispro product, 100 units/mL, demonstrated a mean t1/2 of 0.85 hours (51 minutes) and 0.92 hours (55 minutes), respectively for 0.1 unit/kg and 0.2 unit/kg doses.
Specific Populations
The effects of age, gender, race, obesity, pregnancy, or smoking on the pharmacokinetics of ADMELOG have not been studied.
Patients with renal impairment
Type 2 diabetic patients with varying degrees of renal impairment showed no difference in pharmacokinetics of another insulin lispro product, 100 units/mL. However, the sensitivity of the patients to insulin did change, with an increased response to insulin as the renal function declined. Some studies with human insulin have shown increased circulating levels of insulin in patients with renal impairment. Careful glucose monitoring and dose adjustments of insulin, including ADMELOG, may be necessary in patients with renal dysfunction.
Patients with hepatic impairment
Type 2 diabetic patients with impaired hepatic function showed no effect on the pharmacokinetics of another insulin lispro product, 100 units/mL, as compared to patients with no hepatic dysfunction. However, some studies with human insulin have shown increased circulating levels of insulin in patients with liver failure. Careful glucose monitoring and dose adjustments of insulin, including ADMELOG, may be necessary in patients with hepatic dysfunction.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed. In Fischer 344 rats, a 12-month repeat-dose toxicity study was conducted with insulin lispro at subcutaneous doses of 20 and 200 units/kg/day (approximately 3 and 32 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area). Insulin lispro did not produce important target organ toxicity including mammary tumors at any dose.
Insulin lispro was not mutagenic in the following genetic toxicity assays: bacterial mutation, unscheduled DNA synthesis, mouse lymphoma, chromosomal aberration, and micronucleus assays.
Male fertility was not compromised when male rats given subcutaneous insulin lispro injections of 5 and 20 units/kg/day (0.8 and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area) for 6 months were mated with untreated female rats. In a combined fertility, perinatal, and postnatal study in male and female rats given 1, 5, and 20 units/kg/day subcutaneously (0.16, 0.8, and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area), mating and fertility were not adversely affected in either gender at any dose.
14. Clinical Studies
14.1 Overview of Clinical Studies
The safety and effectiveness of ADMELOG have been established based on adequate and well controlled studies of ADMELOG in adult patients with type 1 and type 2 diabetes mellitus, and based on adequate and well controlled studies of another insulin lispro product, 100 units/mL, in adult and pediatric patients 3 years of age and older with type 1 diabetes mellitus and adult patients with type 2 diabetes mellitus.
The safety and effectiveness of ADMELOG were studied in 507 adult patients with type 1 diabetes and 505 adult patients with type 2 diabetes.
The safety and effectiveness of another insulin lispro product, 100 units/mL, were studied in 1,087 adult and pediatric patients with type 1 diabetes and in 722 adult patients with type 2 diabetes.
14.2 Type 1 Diabetes Mellitus – Subcutaneous Injection
ADMELOG: Study in Adult Patients
A 26-week open-label, active-controlled study (NCT02273180) evaluated the glucose lowering effect of ADMELOG plus insulin glargine, 100 units/mL, compared to that of Comparator (another insulin lispro product, 100 units/mL, or a non–U.S.-approved insulin lispro, 100 units/mL), plus insulin glargine, 100 units/mL. A total of 507 patients with type 1 diabetes mellitus treated with insulin glargine 100 units/mL and rapid-acting mealtime insulin analogs participated in the study. Patients were randomized to ADMELOG (n=253) or Comparator (n=254). ADMELOG or Comparator was administered by subcutaneous injection immediately prior to meals.
The mean age of these subjects was 43 years, and 59.6% were male. The population was 82.1% White, 4.7% Black or African American and 5.3% were Hispanic. The population had type 1 diabetes mellitus for a mean duration of 19 years. The mean eGFR was 90.6 mL/min/1.73 m² and 48.7% of patients had GFR ≥90 mL/min/1.73 m². The mean BMI was approximately 26 kg/m². At baseline, 60.6%, 37.5% and 2.0% of the patients were using other insulin lispro products, 100 units/mL, insulin aspart, 100 units/mL, or both, respectively.
At week 26, treatment with ADMELOG provided a mean reduction in HbA1c that was non-inferior to that achieved with the Comparator (see Table 3).
Table 3. Type 1 Diabetes Mellitus – Adults – Mean Change in HbA1c (ADMELOG plus Insulin Glargine, 100 units/mL, versus Comparator plus Insulin Glargine, 100 units/mL):
| Treatment Duration Treatment in Combination with: | 26 Weeks Insulin Glargine | |
|---|---|---|
| ADMELOG | Comparator | |
| N* | 253 | 254 |
| HbA1c (%) | ||
| Baseline (mean) | 8.08 | 7.99 |
| Adjusted mean change from baseline† | -0.40 | -0.46 |
| Adjusted mean difference‡ (95% CI) | 0.06 (-0.086 to 0.201) | |
* ITT: Intent-to-treat; all randomized patients.
† Estimated using a multiple imputation method that models a "return to baseline" for patients having missing data who discontinued treatment. ANCOVA was used with treatment and stratification groups as fixed factors and baseline HbA1c as a covariate.
‡ Treatment difference: ADMELOG - Comparator.
Another Insulin Lispro Product, 100 units/mL: Study in Adult and Pediatric Patients 12 Years of Age and Older
A 12-month, randomized, parallel, open-label, active-controlled study was conducted in 167 patients with type 1 diabetes to assess the safety and efficacy of another insulin lispro product, 100 units/mL (n=81), compared with regular human insulin, 100 units/mL (n=86). This other insulin lispro product was administered by subcutaneous injection immediately prior to meals and regular human insulin was administered 30 to 45 minutes before meals. Human insulin extended zinc suspension was administered once or twice daily as the basal insulin. There was a 2 to 4-week run-in period with regular human insulin and human insulin extended zinc suspension before randomization.
The mean age of these subjects was 31 years (range 12 to 70 years), and 47% were male. The population was 97% White.
Table 4. Type 1 Diabetes Mellitus – Adults and Pediatric Patients 12 Years of Age and Older – Mean Change in HbA1c% (another insulin lispro product, 100 units/mL, versus regular human insulin, 100 units/mL):
| Treatment Duration Treatment in Combination with: | 12 Months Human Insulin Extended Zinc | |
|---|---|---|
| Another Insulin Lispro Product | Regular Human Insulin | |
| N | 81 | 86 |
| Baseline HbA1c (%)* | 8.2 ± 1.4 | 8.3 ± 1.7 |
| Change from baseline HbA1c (%)* | -0.1 ± 0.9 | 0.1 ± 1.1 |
| Treatment difference in HbA1c mean (95% confidence interval) | 0.4 (0.0; 0.8) | |
* Values are Mean ± SD.
Another Insulin Lispro Product, 100 units/mL: Studies in Pediatric Patients 3 Years of Age and Older
An 8-month, crossover study of pediatric patients with type 1 diabetes (n=463), aged 9 to 19 years, compared two subcutaneous multiple-dose treatment regimens: another insulin lispro product, 100 units/mL, or regular human insulin, 100 units/mL, both administered with NPH human insulin isophane suspension as the basal insulin. Insulin lispro achieved glycemic control comparable to regular human insulin, as measured by HbA1c (see Table 5).
Table 5. Type 1 Diabetes Mellitus – Pediatric Patients 9 Years of Age and Older – Mean Change in HbA1c (%) (another insulin lispro product, 100 units/mL, versus regular human insulin, 100 units/mL):
| Baseline | Another Insulin Lispro Product + NPH | Regular Human Insulin + NPH | |
|---|---|---|---|
| HbA1c (%)* | 8.6 ± 1.5 | 8.7 ± 1.5 | 8.7 ± 1.6 |
| Change from baseline HbA1c (%)* | - | 0.1 ± 1.1 | 0.1 ± 1.3 |
* Values are Mean ± SD.
In a 9-month, crossover study of pediatric patients with type 1 diabetes mellitus (n=60), aged 3 to 11 years, compared three subcutaneous injection regimens: another insulin lispro product, 100 units/mL, administered immediately before meals, this same insulin lispro product, 100 units/mL, administered immediately after meals and regular human insulin, 100 units/mL administered 30 minutes before meals resulted in similar glycemic control, as measured by HbA1c, regardless of treatment group.
14.3 Type 1 Diabetes Mellitus – Continuous Subcutaneous Infusion
Another Insulin Lispro Product, 100 units/mL: Studies in Adult and Pediatric Patients 15 Years of Age and Older
To evaluate the administration of another insulin lispro product, 100 units/mL, as a subcutaneous infusion via external insulin pumps, two open-label, crossover studies were performed in patients with type 1 diabetes mellitus.
One study involved 39 patients, ages 19 to 58 years, treated for 24 weeks with another insulin lispro product, 100 units/mL, or regular human insulin 100 units/mL. After 12 weeks of treatment, the mean HbA1c values decreased from 7.8% to 7.2% in patients treated with another insulin lispro, and from 7.8% to 7.5% in the regular human insulin-treated patients.
Another study involved 60 patients (mean age 39, range 15 to 58 years) treated for 24 weeks with either another insulin lispro product, 100 units/mL, or buffered regular human insulin, 100 units/mL. After 12 weeks of treatment, the mean HbA1c values decreased from 7.7% to 7.4% in patients treated with insulin lispro and remained unchanged from 7.7% in the buffered regular human insulin-treated patients.
Another Insulin Lispro Product, 100 units/mL: Study in Pediatric Patients 4 Years of Age and Older
A randomized, 16-week, open-label, parallel design, study of pediatric patients with type 1 diabetes mellitus (n=298), aged 4 to 18 years, compared two subcutaneous infusion regimens administered via an external insulin pump: insulin aspart, 100 units/mL (n=198), or another insulin lispro product, 100 units/mL (n=100). These two treatments resulted in comparable changes from baseline in HbA1c after 16 weeks of treatment (see Table 6).
Table 6. Type 1 Diabetes Mellitus – Pediatric Patients 4 Years of Age and Older – Mean Change in HbA1c (%) (another insulin lispro product, 100 units/mL, versus insulin aspart, 100 units/mL) in Insulin Pump Study:
| Treatment duration | 16 Weeks | |
|---|---|---|
| Another Insulin Lispro Product | Insulin Aspart | |
| N | 100 | 198 |
| Baseline HbA1c (%)* | 8.2 ± 0.8 | 8.0 ± 0.9 |
| Change from Baseline HbA1c (%) | -0.1 ± 0.7 | -0.1 ± 0.8 |
| Treatment Difference in HbA1c, Mean (95% confidence interval) | 0.1 (-0.3, 0.1) | |
* Values are Mean ± SD.
14.4 Type 2 Diabetes Mellitus
ADMELOG: Study in Adult Patients
A 26-week open-label, active-controlled study (NCT02294474) evaluated the glucose lowering effect of ADMELOG plus insulin glargine, 100 units/mL, compared to that of Comparator (another insulin lispro product, 100 units/mL, or a non–U.S.-approved insulin lispro, 100 units/mL) plus insulin glargine, 100 units/mL. A total of 505 patients with type 2 diabetes mellitus treated with insulin glargine, 100 units/mL, and rapid-acting mealtime insulin analogs participated in the study. Patients were randomized to ADMELOG, 100 units/mL (n=253) or Comparator (n=252). ADMELOG or Comparator, was administered by subcutaneous injection immediately prior to meals.
The mean age of these subjects was 62.5 years, and 53.1% were male. The population was 88.3% White, 6.1% Black or African American and 17.8% were Hispanic. The population had type 2 diabetes mellitus for a mean duration of 17 years. The mean eGFR was 77.9 mL/min/1.73 m² and 26.9% of patients had GFR >90 mL/min/1.73 m². The mean BMI was approximately 32.2 kg/m². At baseline, 51.4%, 48.2%, and 0.4% of the patients were using other insulin lispro products, 100 units/mL, insulin aspart, 100 units/mL, or both, respectively.
At week 26, treatment with ADMELOG provided a mean reduction in HbA1c that was non-inferior to that achieved with the Comparator (see Table 7).
Table 7. Type 2 Diabetes Mellitus – Adults – Mean Change in HbA1c (%) (ADMELOG plus insulin glargine, 100 units/mL, versus comparator plus insulin glargine, 100 units/mL):
| Treatment Duration Treatment in Combination with: | 26 Weeks Insulin Glargine | |
|---|---|---|
| ADMELOG | Comparator | |
| N* | 253 | 252 |
| HbA1c (%) | ||
| Baseline (mean) | 8.00 | 8.03 |
| Adjusted mean change from baseline† | -0.86 | -0.80 |
| Adjusted mean difference‡ (95% CI) | -0.06 (-0.209 to 0.091) | |
* ITT: Intent-to-treat; all randomized patients.
† Estimated using a multiple imputation method that models a "return-to-baseline" for patients having missing data who discontinued treatment. ANCOVA was used with treatment and stratification groups as fixed factors and baseline HbA1c as a covariate.
‡ Treatment difference: ADMELOG – Comparator.
Another Insulin Lispro Product, 100 units/mL: Study in Adult Patients
A 6-month randomized, crossover, open-label, active-controlled study was conducted in 722 patients with type 2 diabetes mellitus treated with insulin to assess the safety and efficacy of another insulin lispro product, 100 units/mL, for 3 months followed by regular human insulin, 100 units/mL, for 3 months or the reverse sequence. This other insulin lispro product was administered by subcutaneous injection immediately before meals and regular human insulin was administered 30 to 45 minutes before meals. NPH human insulin isophane suspension or human insulin extended zinc suspension was administered once or twice daily as the basal insulin. All patients participated in a 2 to 4-week run-in period with regular human insulin and NPH human insulin isophane suspension or human insulin extended zinc suspension.
Most of the patients were Caucasian (88%), and the numbers of men and women in each group were approximately equal. The mean age was 58.6 years (range 23.8 to 85 years). The average body mass index (BMI) was 28.2 kg/m². During the study, the majority of patients used NPH human insulin isophane suspension (84%) compared with human insulin extended zinc suspension (16%) as their basal insulin. The reductions from baseline in HbA1c were similar between the two treatments from the combined groups (see Table 8).
Table 8. Type 2 Diabetes Mellitus – Adults – Mean Change in HbA1c (%) (another insulin lispro product, 100 units/mL, versus regular human insulin, 100 units/mL):
| Treatment Duration | 3 Months | ||
|---|---|---|---|
| Baseline | Another Insulin Lispro Product + Basal | Regular Human Insulin + Basal | |
| HbA1c (%)* | 8.9 ± 1.7 | 8.2 ± 1.3 | 8.2 ± 1.4 |
| Change from baseline HbA1c (%)* | - | -0.7 ± 1.4 | -0.7 ± 1.3 |
* Values are Mean ± SD.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.