ADRENALINE AGUETTANT 1 mg/10 ml Solution for injection in pre-filled syringe Ref.[7553] Active ingredients: Epinephrine
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Laboratoire Aguettant, 1 rue Alexander Fleming, 69007 LYON, FRANCE
Therapeutic indications
Cardiopulmonary resuscitation.
Acute anaphylaxis in adults.
Posology and method of administration
Intravenous adrenaline should only be administered by those experienced in the use and titration of vasopressors in their normal clinical practice.
Cardiopulmonary resuscitation
10 ml of the 1:10,000 solution (1 mg) by the intravenous or intraosseous route, repeated every 3-5 minutes until return of spontaneous circulation.
Endotracheal use should only be considered as a last resort if no other route of administration is accessible, at a dose of 20 to 25 ml of the 1:10,000 solution (2 to 2.5 mg).
In cardiac arrest following cardiac surgery, Adrenaline should be administered intravenously in doses of 0.5 ml or 1 ml of 1:10,000 solution (50 or 100 micrograms) very cautiously and titrated to effect.
Acute anaphylaxis
Titrate using intravenous boluses of 0.5 ml 1:10,000 solution (0.05 mg) according to response.
Adrenaline 1 mg/10 ml (1:10,000) solution for injection in pre-filled syringe is not recommended for intramuscular use in acute anaphylaxis. For intramuscular administration, a 1 mg/ml (1:1000) solution should be used.
Paediatric Population
This medicinal product is not suitable for delivering a dose of less than 0.5 ml and should therefore not be used by the intravenous or intraosseous route, in neonates and infants with body weight less than 5 kg.
Cardiac arrest in children
Intravenous or intraosseous route (above 5 kg only): 0.1 ml/kg of 1:10,000 solution (10 micrograms/kg) to a maximum single dose of 10 ml of 1:10,000 solution (1 mg), repeated every 3-5 minutes until return of spontaneous circulation.
Endotracheal use (any body weight) should only be considered as a last resort if no other route of administration is accessible, at a dose of 1 ml/kg of 1:10,000 solution (100 micrograms/kg) to a maximum of single dose of 25 ml of 1:10,000 solution (2.5 mg).
Overdose
Over dosage or inadvertent intravenous administration of adrenaline may produce severe hypertension. Cerebral, cardiac or vascular accidents which could be potentially fatal may occur as a result (cerebral haemorrhage, dysrhythmias such as transient bradycardia followed by tachycardia that may result in arrhythmia, myocardial necrosis, acute pulmonary oedema, renal insufficiency).
The effects of adrenaline may be counteracted, depending on the condition of the patient, by administration of quick-acting vasodilators, of quick-acting alpha-adrenoreceptor blocking agents (e.g. phentolamine), or beta-adrenoreceptor blocking agents (e.g. propanolol). However, due to the short half-life of adrenaline, treatment with these medicines may not be necessary. In case of prolonged hypotensive reaction, administration of another vasopressive agent such as noradrenaline may be required.
Shelf life
Unopened: 24 months.
Special precautions for storage
Do not store above 25°C.
Do not freeze.
Store in the aluminium pouch in order to protect from light and oxygen.
Nature and contents of container
10 ml solution in a polypropylene pre-filled syringe without a needle, individually packaged in a transparent blister and overwrapped in an aluminium pouch containing an oxygen absorbing sachet. Available in a box of 1 or 10.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The aluminium pouch and syringe blister should only be opened immediately prior administration.
After opening the pouch the product must be used immediately.
The external surface of the syringe and its content are sterile if the blister is unopened and undamaged.
Strictly respect the protocol below:
The pre-filled syringe is for single patient use only. Discard the syringe after use. Do not reuse.
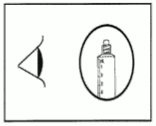
The product should be inspected visually for particles and discolouration prior to administration. Only clear colourless solution free from particles or precipitates should be used.
The product should not be used if the pouch or the blister has been opened or if the tamper evident seal on the syringe (plastic film at the basis of the end cap) is broken.
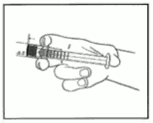
1) Tear open the aluminium pouch by hand only using the indent(s) Do not use sharp instruments to open the pouch.
2) Withdraw the pre-filled syringe from the sterile blister.
3) Push on the plunger to free the bung. The sterilisation process may have caused adhesion of the bung to the body of the syringe.
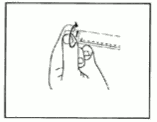
4) Twist off the end cap to break the seals. Do not touch the exposed luer connection in order to avoid contamination.
5) Check the syringe seal tip has been completely removed.
If not, replace the cap and twist again.
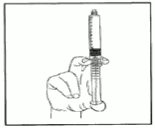
6) Expel the air by gently pushing the plunger.
7) Connect syringe to vascular access device or to needle. Push the plunger to inject the required volume.
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.