ALOXI Soft capsule Ref.[8703] Active ingredients: Palonosetron
Source: European Medicines Agency (EU) Revision Year: 2018 Publisher: Helsinn Birex Pharmaceuticals Limited, Damastown, Mulhuddart, Dublin 15, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Antiemetics and antinauseants, Serotonin (5HT3) antagonists
ATC code: A04AA05
Palonosetron is a selective high-affinity receptor antagonist of the 5HT3 receptor.
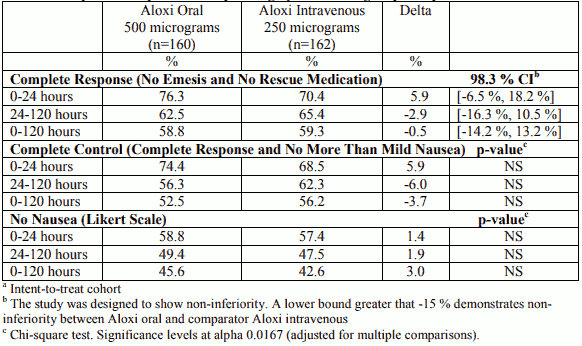
In a multicentre, randomised, double-blind active control clinical trial of 635 patients set to receive moderately emetogenic cancer chemotherapy. A single-dose of 250 mcg, 500 mcg, or 750 mcg oral palonosetron capsules given one hour prior to moderately emetogenic chemotherapy was compared to a single-dose of 250 mcg intravenous Aloxi given 30 minutes prior to chemotherapy. Patients were randomised to either dexamethasone or placebo in addition to their assigned treatment. The majority of patients in the study were women (73%), white (69%), and naïve to previous chemotherapy (59%). The antiemetic activity was observed during 0-24 hours, 24-120 hours and 0-120 hours. Efficacy was based on demonstrating non-inferiority of oral palonosetron doses compared to the approved intravenous formulation. Non-inferiority criteria were met if the lower bound of the twosided 98.3% confidence interval for the difference in complete response rates of oral palonosetron dose minus approved intravenous formulation was larger than -15%. The non-inferiority margin was 15%.
As shown in Table 1, oral Aloxi capsules 500 micrograms demonstrated non-inferiority to the active comparator during the 0 to 24 hour and 0 to 120 hour time intervals; however, for the 24 to 120 hour time period, non-inferiority was not shown.
Although comparative efficacy of palonosetron in multiple cycles has not been demonstrated in controlled clinical trials, 217 patients were enrolled in a multicentre, open label safety study and were treated with palonosetron capsules 750 micrograms for up to 4 cycles of chemotherapy in a total of 654 chemotherapy cycles. Approximately 74% of patients also received single dose oral or intravenous dexamethasone 30 minutes before chemotherapy. Complete Response was not formally evaluated for the repeat cycle application. However, in general the antiemetic effect for the 0-24 hour interval was similar throughout the consecutively repeated cycles and the overall safety was maintained during all cycles.
Table 1. Proportion of patientsa responding by treatment group and phase:
In non-clinical studies palonosetron possesses the ability to block ion channels involved in ventricular de- and re-polarisation and to prolong action potential duration. The effect of palonosetron on QTc interval was evaluated in a double blind, randomised, parallel, placebo and positive (moxifloxacin) controlled trial in adult men and women. The objective was to evaluate the ECG effects of IV administered palonosetron at single doses of 0.25, 0.75 or 2.25 mg in 221 healthy subjects. The study demonstrated no effect on QT/QTc interval duration as well as any other ECG interval at doses up to 2.25 mg. No clinically significant changes were shown on heart rate, atrioventricular (AV) conduction and cardiac repolarization.
Paediatric population
Prevention of Chemotherapy Induced Nausea and Vomiting (CINV)
The safety and efficacy of Palonosetron i.v at single doses of 3µg/kg and 10µg/kg was investigated in the first clinical study in 72 patients in the following age groups, >28 days to 23 months (12 patients), 2 to 11 years (31 patients), and 12 to 17 years of age (29 patients), receiving highly or moderately emetogenic chemotherapy. No safety concerns were raised at either dose level. The primary efficacy variable was the proportion of patients with a complete response (CR, defined as no emetic episode and no rescue medication) during the first 24 hours after the start of chemotherapy administration. Efficacy after palonosetron 10 µg/kg compared to palonosetron 3µg/kg was 54.1% and 37.1% respectively.
The efficacy of Aloxi for the prevention of chemotherapy-induced nausea and vomiting in paediatric cancer patients was demonstrated in a second non-inferiority pivotal trial comparing a single intravenous infusion of palonosetron versus an i.v. ondansetron regimen. A total of 493 paediatric patients, aged 64 days to 16.9 years, receiving moderately (69.2%) or highly emetogenic chemotherapy (30.8%) were treated with palonosetron 10 µg/kg (maximum 0.75 mg), palonosetron 20 µg/kg (maximum 1.5 mg) or ondansetron (3 × 0.15 mg/kg , maximum total dose 32 mg) 30 minutes prior to the start of emetogenic chemotherapy during Cycle 1. Most patients were non-naïve to chemotherapy (78.5%) across all treatment groups. Emetogenic chemotherapies administered included doxorubicin, cyclophosphamide (<1500 mg/m²), ifosfamide, cisplatin, dactinomycin, carboplatin, and daunorubicin. Adjuvant corticosteroids, including dexamethasone, were administered with chemotherapy in 55% of patients. The primary efficacy endpoint was Complete Response in the acute phase of the first cycle of chemotherapy, defined as no vomiting, no retching, and no rescue medication in the first 24 hours after starting chemotherapy. Efficacy was based on demonstrating non-inferiority of intravenous palonosetron compared to intravenous ondansetron. Non-inferiority criteria were met if the lower bound of the 97.5% confidence interval for the difference in Complete Response rates of intravenous palonosetron minus intravenous ondansetron was larger than -15%. In the palonosetron 10 µg/kg, 20 µg/kg and ondansetron groups, the proportion of patients with CR0-24h was 54.2%, 59.4% and 58.6%. Since the 97.5% confidence interval (stratum adjusted MantelHaenszel test) of the difference in CR0-24h between palonosetron 20 µg/kg and ondansetron was [-11.7%, 12.4%], the 20 µg/kg palonosetron dose demonstrated non-inferiority to ondansetron.
While this study demonstrated that paediatric patients require a higher palonosetron dose than adults to prevent chemotherapy-induced nausea and vomiting, the safety profile is consistent with the established profile in adults (see section 4.8). Pharmacokinetic information is provided in section 5.2.
Prevention of Post Operative Nausea and Vomiting (PONV)
Two paediatric trials were performed. The safety and efficacy of Palonosetron i.v at single doses of 1µg/kg and 3µg/kg was compared in the first clinical study in 150 patients in the following age groups, >28 days to 23 months (7 patients), 2 to 11 years (96 patients), and 12 to 16 years of age (47 patients) undergoing elective surgery. No safety concerns were raised in either treatment group. The proportion of patients without emesis during 0-72 hours post-operatively was similar after palonosetron 1 µg/kg or 3 µg/kg (88% vs 84%).
The second paediatric trial was a multicenter, double-blind, double-dummy, randomised, parallel group, active control, single-dose non-inferiority study, comparing i.v. palonosetron (1 µg/kg, max 0.075 mg) versus i.v. ondansetron. A total of 670 paediatric surgical patients participated, age 30 days to 16.9 years. The primary efficacy endpoint, Complete Response (CR: no vomiting, no retching, and no antiemetic rescue medication) during the first 24 hours postoperatively was achieved in 78.2% of patients in the palonosetron group and 82.7% in the ondansetron group. Given the pre-specified noninferiority margin of -10%, the stratum adjusted Mantel-Haenszel statistical non-inferiority confidence interval for the difference in the primary endpoint, complete response (CR), was [-10.5, 1.7%], therefore non-inferiority was not demonstrated No new safety concerns were raised in either treatment group.
Please see section 4.2 for information on paediatric use.
Pharmacokinetic properties
Absorption
Following oral administration, palonosetron is well absorbed with its absolute bioavailability reaching 97%. After single oral doses using buffered solution mean maximum palonosetron concentrations (Cmax) and area under the concentration-time curve (AUC0-∞) were dose proportional over the dose range of 3.0 to 80 µg/kg in healthy subjects.
In 36 healthy male and female subjects given a single oral dose of palonosetron capsules 500 micrograms, maximum plasma palonosetron concentration (Cmax) was 0.81±0.17 ng/ml (mean±SD) and time to maximum concentration (Tmax) was 5.1±1.7 hours. In female subjects (n=18), the mean AUC was 35% higher and the mean Cmax was 26% higher than in male subjects (n=18). In 12 cancer patients given a single oral dose of palonosetron capsules 500 micrograms one hour prior to chemotherapy, Cmax was 0.93±0.34 ng/ml and Tmax was 5.1±5.9 hours. The AUC was 30% higher in cancer patients than in healthy subjects.
A high fat meal did not affect the Cmax and AUC of oral palonosetron. Therefore, Aloxi capsules may be taken without regard to meals.
Distribution
Palonosetron at the recommended dose is widely distributed in the body with a volume of distribution of approximately 6.9 to 7.9 l/kg. Approximately 62% of palonosetron is bound to plasma proteins.
Biotransformation
Palonosetron is eliminated by dual route, about 40% eliminated through the kidney and with approximately 50% metabolised to form two primary metabolites, which have less than 1% of the 5HT3 receptor antagonist activity of palonosetron. In vitro metabolism studies have shown that CYP2D6 and to a lesser extent, CYP3A4 and CYP1A2 isoenzymes are involved in the metabolism of palonosetron. However, clinical pharmacokinetic parameters are not significantly different between poor and extensive metabolisers of CYP2D6 substrates. Palonosetron does not inhibit or induce cytochrome P450 isoenzymes at clinically relevant concentrations.
Elimination
Following administration of a single oral 750 micrograms dose of [14C]-palonosetron to six healthy subjects, 85% to 93% of the total radioactivity was excreted in urine, and 5% to 8% was eliminated in feces. The amount of unchanged palonosetron excreted in the urine represented approximately 40% of the administered dose. In healthy subjects given palonosetron capsules 500 micrograms, the terminal elimination half-life (t½) of palonosetron was 37±12 hours (mean±SD), and in cancer patients, t½ was 48±19 hours. After a single-dose of approximately 0.75 mg intravenous palonosetron, the total body clearance of palonosetron in healthy subjects was 160±35 ml/h/kg (mean±SD) and renal clearance was 66.5±18.2 ml/h/kg.
Pharmacokinetics in special populations
Elderly people
Age does not affect the pharmacokinetics of palonosetron. No dose adjustment is necessary in elderly patients.
Gender
Gender does not affect the pharmacokinetics of palonosetron. No dose adjustment is necessary based on gender.
Paediatric patients
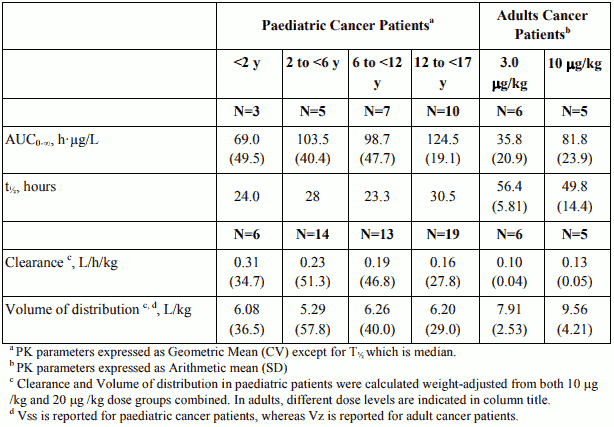
Single-dose i.v. Aloxi pharmacokinetic data was obtained from a subset of paediatric cancer patients (n=280) that received 10 µg/kg or 20 µg/kg. When the dose was increased from10 µg/kg to 20 µg/kg a dose-proportional increase in mean AUC was observed. Following single dose intravenous infusion of Aloxi 20 µg/kg, peak plasma concentrations (CT) reported at the end of the 15 minute infusion were highly variable in all age groups and tended to be lower in patients <6 years than in older paediatric patients. Median half-life was 29.5 hours in overall age groups and ranged from about 20 to 30 hours across age groups after administration of 20 µg/kg.
The total body clearance (L/h/kg) in patients 12 to 17 years old was similar to that in healthy adults. There are no apparent differences in volume of distribution when expressed as L/kg.
Table 2. Pharmacokinetic Parameters in Paediatric Cancer Patients following intravenous infusion of Aloxi at 20 µg/kg over 15 min and in Adult Cancer Patients receiving 3 and 10 µg/kg palonosetron doses via intravenous bolus:
Renal impairment
Mild to moderate renal impairment does not significantly affect palonosetron pharmacokinetic parameters. Severe renal impairment reduces renal clearance, however total body clearance in these patients is similar to healthy subjects. No dose adjustment is necessary in patients with renal insufficiency. No pharmacokinetic data in haemodialysis patients are available.
Hepatic impairment
Hepatic impairment does not significantly affect total body clearance of palonosetron compared to the healthy subjects. While the terminal elimination half-life and mean systemic exposure of palonosetron is increased in the subjects with severe hepatic impairment, this does not warrant dose reduction.
Preclinical safety data
Effects in non-clinical studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use.
Non-clinical studies indicate that palonosetron, only at very high concentrations, may block ion channels involved in ventricular de- and re-polarisation and prolong action potential duration.
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development. Only limited data from animal studies are available regarding the placental transfer (see section 4.6).
Palonosetron is not mutagenic. High doses of palonosetron (each dose causing at least 15 times the human therapeutic exposure) applied daily for two years caused an increased rate of liver tumours, endocrine neoplasms (in thyroid, pituitary, pancreas, adrenal medulla) and skin tumours in rats but not in mice. The underlying mechanisms are not fully understood, but because of the high doses employed and since Aloxi is intended for single application in humans, these findings are not considered relevant for clinical use.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.