ALREX Ophthalmic suspension Ref.[10745] Active ingredients: Loteprednol
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
ALREX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.
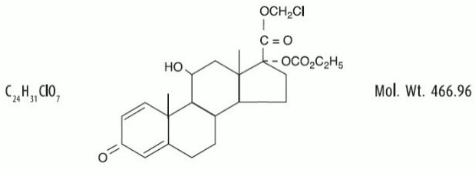
Loteprednol etabonate is represented by the following structural formula:
Chemical Name:
chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylate
Each mL contains:
ACTIVE: Loteprednol Etabonate 2 mg (0.2%);
INACTIVES: Edetate Disodium, Glycerin, Povidone, Purified Water and Tyloxapol. Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust the pH. The suspension is essentially isotonic with a tonicity of 250 to 310 mOsmol/kg.
PRESERVATIVE ADDED: Benzalkonium Chloride 0.01%.
| How Supplied |
|---|
|
ALREX (loteprednol etabonate ophthalmic suspension, 0.2%) is supplied in a plastic bottle with a controlled drop tip in the following sizes: NDC 24208-353-05 5 mL in a 7.5 mL bottle Distributed by: Bausch + Lomb, a division of Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Bausch and & Lomb Incorporated, Tampa, FL 33637 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ALREX | Brazil, Canada, Hong Kong, Singapore, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.